Abstract
Research Article
Oral Candida colonization in HIV-infected patients: Species and antifungal susceptibility in Tripoli/Libya
Ellabib M*, Mohamed H, Mokthar E, Ellabib M, El Magrahi H and Eshwika A
Published: 03 August, 2018 | Volume 1 - Issue 1 | Pages: 001-008
Introduction: Candidiasis is more frequent in human immunodeficiency virus (HIV)-infected patients and knowledge about the distribution and antifungal susceptibility of oral Candida species is important for effective management of candidiasis.
Material and Methods: An oral rinses sample collected from hundred HIV-infected patients with and without clinical evidence of oral candidiasis in this study at the Infectious Department/Tripoli Medical Center, Libya. Species identified by standard phenotypic and conventional methods and in vitro susceptibility testing of the yeast isolates to antifungals were performed using the Disc diffusion method protocol as recommended by the Clinical Laboratory Scientific Institute.
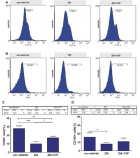
Results: Oral Candida colonization is detected in all patients with and without clinical syndromes, and Candida albicans were accounted for (74%), C. dubliniensis (11%) and C. glabrata (6%). A high proportion of Candida species (42%) showed decreased susceptibility to fluconazole. Among C., albicans more than 30% of isolate were resistant to most new azole antifungal including fluconazole, itraconazole, posoconazole and voriconazole.
Conclusions: A significant number of oral Candida species particular Candida albicans exhibiting decreased susceptibility to fluconazole were isolated from colonized HIV-infected individual, given the high incidence and severity of fungal infections in HIV-infected individuals. The results of this study reinforce the importance of antifungal susceptibility testing, which contributes to the therapeutic strategies and highlights the need for continuous surveillance of Candida colonization in this population.
Read Full Article HTML DOI: 10.29328/journal.ijcmbt.1001001 Cite this Article Read Full Article PDF
Keywords:
Candida; HIV; Azole resistance
References
- William JD, Timothy BG. Andrews' diseases of the skin: Clinical dermatology. Saunders Elsevier. 2006; 45.
- Crispian S. Oral and maxillofacial medicine: The basis of diagnosis and treatment (2nd ed). Edinburgh: Churchill Livingstone. 2008; 191-199.
- Fidel PL. Candida-host interactions in HIV disease: Relationships in oropharyngeal candidiasis. Adv Dent Res. 2006; 19: 80-84. Ref.: https://tinyurl.com/ybo4n8mn
- Mousavi SAA, Salari S, Rezaie S, Nejad NS, Hadizadeh S, et al. Identification of Candida species isolated from oral colonization in Iranian HIV-positive patients, by PCR-RFLP method. Jundishapur J Microbiol. 2012; 5: 336-340. Ref.: https://tinyurl.com/ya6sa46j
- Wabe NT, Hussein J, Suleman S, Abdella K. In vitro antifungal susceptibility of Candida albicans isolates from oral cavities of patients infected with human immunodeficiency virus in Ethiopia. J Exper and Integra Med. 2011; 1: 265-271. Ref.: https://tinyurl.com/yak5a2w4
- Khan AP, Malik A, Khan SH. Profile of candidiasis in HIV infected patients. Ira J of Microiol. 2012; 4: 204-209. Ref.: https://tinyurl.com/ycpztjby
- M Vajpayee, S Kanswal, P Seth, N Wig. Spectrum of Opportunistic Infections and Profile of CD4+Counts among AIDS Patients in North India. Infect. 2003; 31: 336-340. Ref.: https://tinyurl.com/ydxly745
- Martins MD, Lozano-Chiu M, Rex JH. Point prevalence of oropharyngeal colonization of fluconazole-resistant Candida in human immunodeficiency virus-infected patients. Clin Infect Dis. 1997; 25: 843-846. Ref.: https://tinyurl.com/y7omeuku
- Michelet C, Arvieux C, François C, Besnier JM, Rogez JP, et al. Opportunistic infections occurring during highly active antiretroviral treatment. AIDS. 1998; 12: 1815-1822. Ref.: https://tinyurl.com/ybw86yxp
- Tooyama H, Matsumoto T, Hayashi K, Kurashina K, Kurita H, et al. Candida concentrations determined following concentrated oral rinse culture reflect clinical oral signs. BMC Oral Health. 2015; 15: 150. Ref.: https://tinyurl.com/ybdpow64
- Jan A, Bashir G, Fomda BA, Khangsar DA, Manzoor M, et al. Hypertonic xylose agar medium: A novel medium for differentiation of Candida dubliniensis from Candida albicans. Indian J Med Microbiol. 2018; 35: 518-521. Ref.: https://tinyurl.com/y79ajhrq
- Khan ZU, Ahmad S, Mokaddas E, Chandy R. Simplified sunflower (Helianthus annuus) seed agar for differentiation of Candida dubliniensis from Candida albicans. Clin Microbiol Infect. 2004; 10: 590-592. Ref.: https://tinyurl.com/y75v62ww
- Clinical and Laboratory Standards Institute (CLSI). Method for antifungal disk diffusion susceptibility testing of yeasts; approved guideline-2nd ed. CLSI document M44-A2. Wayne. PA: CLSI. 2009.
- Clinical and Laboratory Standards Institute (CLSI). Zone diameter interpretive standards, corresponding minimal inhibitory concentration (MIC) interpretive breakpoints, and quality control limits for antifungal disk diffusion susceptibility testing of yeasts, Third International Supplement CLSI document-M44-S3. Wayne. PA: CLSI. 2009.
- De Resende MA, de Sousa LV, de Oliveira RC, Koga-Ito CY, Lyon JP. Prevalence and Antifungal Susceptibility of Yeasts Obtained from the Oral Cavity of Elderly Individuals. Mycopathologia. 2006; 162: 39-44. Ref.: https://tinyurl.com/y9exh4v8
- Wabale V, Kagal A, Bharadwaj R. Characterization of Candida spp. from oral thrush in human immunodeficiency virus (HIV) seropositive and seronegative patients. Bombay Hospital J. 2008; 50: 212-217. Ref.: https://tinyurl.com/ya9j3esx
- Baradkar VP, Kumar S. Species identification of Candida isolates obtained from oral lesions of HIV infected patients. Indian J Dermatol. 2009; 54: 385-386. Ref.: https://tinyurl.com/y9vrzx2s
- Lattif AA, Uma B, Rajendra P, Biswas A, Wig N, et al. Susceptibility pattern and molecular type of speciesspecific Candida in oropharyngeal lesions of Indian human immunodeficiency virus-positive patients. J Clin Microbiol. 2004; 42: 1260-1262. Ref.: https://tinyurl.com/ycx525bg
- Chunchanur SK, Nadgir SD, Halesh LH, Patil BS, Kausar Y, et al. Detection and antifungal susceptibility testing of oral Candida dubliniensis from human immunodeficiency virus-infected patients. Indian J Pathol Microbiol. 2009; 52: 501-504. Ref.: https://tinyurl.com/y9vco8s3
- Campisi G, Pizzo G, Milici M, Mancuso S, Margiotta V. Candidal colonization in the oral cavity of human immunodeficiency virus-infected subjects. Oral Surg Oral Med Oral Path Oral Radiol Oral Endod. 2002; 93: 281-286. Ref.: https://tinyurl.com/y8ntoyka
- Ellabib MS, ElJariny IA. In vitro activity of 6 antifungal agents on candida species isolated as causative agents from vaginal and other clinical specimens. Saudi Med J. 2001; 22: 860-863. Ref.: https://tinyurl.com/y9e5l9l4
- Krema ZA, Trfas EEM, Ellabib MS, Cogliati M. Oral colonization of candidiasis in patients with oral dental diseases: predisposing factors, species and their antifungal susceptibility patterns. Journal of Bacteriology & Mycology: Open Access. 2018; 6: 223-227.
- Arora U, Jagdev M, Jindal N. Immunosuppression level in HIV-positive patients with oropharyngeal Candidiasis. Indian J Med Microbiol. 2009; 27: 174-175. Ref.: https://tinyurl.com/yaxz8yom
- Costa CR, de Lemos JA, Passos XS, de Arau´jo CR, Cohen AJ, et al. Species distribution and antifungal susceptibility profile of oral Candida isolates from HIV-infected patients in the antiretroviral therapy era. Mycopathologia. 2006; 162: 45-50. Ref.: https://tinyurl.com/y76wryqg
- EYESON JD, TENANT-FLOWERS M, COOPER DJ, NW Johnson, KAAS Warnakulasuriya. Oral manifestations of an HIV positive cohort in the era of highly active anti-retroviral therapy (HAART) in South London. J. oral Path. Med. 2002; 31: 169-174. Ref.: https://tinyurl.com/y855q52x
- Staszewski S1, Loveday C, Picazo JJ, Dellarnonica P, Skinhøj P, et al. Safety and efficacy of lamivudine-zidovudine combination therapy in zidovudine-experienced patients. A randomized controlled comparison with zidovudine monotherapy. Lamivudine European HIV Working Group. JAMA. 1996; 276: 111-117. Ref.: https://tinyurl.com/ycnf6haw
- Nicolatou-galitis O, Velegraki A, Paikos S, Economopoulou P, Stefaniotis T, et al. Effect of pi-haart on the prevalence of oral lesions in HIV-I infected patients. A Greek study. Oral Dis. 2004; 10: 145-150. Ref.: https://tinyurl.com/y8mlnu29
- Gugnani HC, Becker K, Fegeler W, Basu S, Chattopadhya D, et al. Oropharyngeal colonization of Candida species in HIV-infected patients in India. Mycoses. 2003; 46: 299-306. Ref.: https://tinyurl.com/y745fuqs
- Yang YL, Lo HJ, Hung CC, Li Y. Effect of prolonged HAART on oral colonization with Candida and candidiasis. BMC Infect Dis. 2006; 6: 8. Ref.: https://tinyurl.com/yc6622f8
- Sanchez-Vargas LO, Ortiz-Lopez NG, Villar M, Moragues MD, Aguirre JM, et al. Point prevalence, microbiology and antifungal susceptibility patterns of oral Candida isolates colonizing or infecting Mexican HIV/AIDS patients and healthy persons. Rev Iberoam Micol. 2005; 22: 83-92. Ref.: https://tinyurl.com/y92stslu
- Vijeta Maurya, Ashutosh Srivastava, Jyoti Mishra, Rajni Gaind, Rungmei SK Marak, et al. Oropharyngeal candidiasis and Candida colonization in HIV positive patients in northern India. J Infect Dev Ctries. 2013; 7: 608-613. Ref.: https://tinyurl.com/ycy4nunm
- Sangeorzan JA, Bradley SF, He X, Lidija Zarins T, George Ridenour L, et al. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med. 1994; 97: 339-346. Ref.: https://tinyurl.com/y8f3vqt3
- Sánchez-Vargas LO, Ortiz-López NG, Villar M, María Dolores Moragues, José Manuel Aguirre, et al. Oral Candida isolates colonizing or infecting human immunodeficiency virus-infected and healthy persons in Mexico. J Clin Microbiol. 2005; 43: 4159-4162. Ref.: https://tinyurl.com/yajt6aw9
- Thomas Campbell B, Laura Smeaton M, Kumarasamy N, Timothy Flanigan, Karin Klingman L, et al. Efficacy and Safety of Three Antiretroviral Regimens for Initial Treatment of HIV-1: A Randomized Clinical Trial in Diverse Multinational Settings. PLOS Med. 2012; 9: e1001290. Ref.: https://tinyurl.com/ycty3qrh
Similar Articles
-
Oral Candida colonization in HIV-infected patients: Species and antifungal susceptibility in Tripoli/LibyaEllabib M*,Mohamed H,Mokthar E,Ellabib M,El Magrahi H,Eshwika A. Oral Candida colonization in HIV-infected patients: Species and antifungal susceptibility in Tripoli/Libya . . 2018 doi: 10.29328/journal.ijcmbt.1001001; 1: 001-008
-
Host biomarkers for early diagnosis of infectious diseases: A comprehensive reviewArindam Chakraborty*,Singh Monica. Host biomarkers for early diagnosis of infectious diseases: A comprehensive review. . 2019 doi: 10.29328/journal.ijcmbt.1001005; 2: 001-007
-
Atherogenic risk assessment of naive HIV-infected patients attending Infectious Diseases Service of Kinshasa University Teaching Hospital, Democratic Republic of the Congo (DRC)MMK Mbula*,HNT Situakibanza,GL Mananga,B Longo Mbenza, JRR Makulo,MM Longokolo,MN Mandina,NN Mayasi,MM Mbula,B Bepouka,GL Mvumbi,EN Amaela,DN Tshilumba,O Odio,BM Ekila,A Nkodila,BT Buasa. Atherogenic risk assessment of naive HIV-infected patients attending Infectious Diseases Service of Kinshasa University Teaching Hospital, Democratic Republic of the Congo (DRC). . 2020 doi: 10.29328/journal.ijcmbt.1001015; 3: 040-048
-
The clinicopathological correlates of Cystoisosporiasis in immunocompetent, immunocompromised and HIV-infected/AIDS patients, but neglected in SARS-COV-2/COVID-19 patients?Chrysanthus Chukwuma Sr*. The clinicopathological correlates of Cystoisosporiasis in immunocompetent, immunocompromised and HIV-infected/AIDS patients, but neglected in SARS-COV-2/COVID-19 patients?. . 2021 doi: 10.29328/journal.ijcmbt.1001018; 4: 001-004
-
The Bacteriological Profile of Nosocomial Infections at the Army Central Hospital of BrazzavilleMedard Amona*,Yolande Voumbo Matoumona Mavoungou,Hama Nemet Ondzotto,Benjamin Kokolo,Armel Itoua,Gilius Axel Aloumba,Pascal Ibata. The Bacteriological Profile of Nosocomial Infections at the Army Central Hospital of Brazzaville. . 2025 doi: 10.29328/journal.ijcmbt.1001032; 8: 009-022
-
Analysis of the Factors of Therapeutic Failure after Transition to Acriptega: About Two CasesMedard Amona*,Yolande Voumbo Mavoungou Matoumona,Hama Nemet Ondzotto,Grace Paterson Ngouaka,Benjamin Kokolo,Armel Itoua,Gilius Axel Aloumba,Pascal Ibata. Analysis of the Factors of Therapeutic Failure after Transition to Acriptega: About Two Cases. . 2026 doi: 10.29328/journal.ijcmbt.1001033; 9: 001-009
Recently Viewed
-
Deep Learning-Powered Genetic Insights for Elite Swimming Performance: Integrating DNA Markers, Physiological Biometrics and Performance AnalyticsRahul Kathuria,Reeta Devi,Asadi Srinivasulu*. Deep Learning-Powered Genetic Insights for Elite Swimming Performance: Integrating DNA Markers, Physiological Biometrics and Performance Analytics. Int J Bone Marrow Res. 2025: doi: 10.29328/journal.ijbmr.1001020; 8: 006-015
-
Screening for Depressive Symptoms in Clinical and Nonclinical Youth: The Psychometric Properties of the Dutch Children’s Depression Inventory-2 (CDI-2)Denise HM Bodden*,Yvonne Stikkelbroek,Daan Creemers,Sanne PA Rasing,Elien De Caluwe,Caroline Braet. Screening for Depressive Symptoms in Clinical and Nonclinical Youth: The Psychometric Properties of the Dutch Children’s Depression Inventory-2 (CDI-2). Insights Depress Anxiety. 2025: doi: 10.29328/journal.ida.1001047; 9: 028-039
-
Stone on the Mesh: Intravesical Erosion after Laparoscopic Promontofixation-A Hidden Cost of DurabilityAyoub Mamad*,Mohammed Amine Bibat,Mohammed Amine Elafari,Midaoui Moncef,Amine Slaoui,Tarik Karmouni,Abdelatif Koutani,Khalid Elkhader. Stone on the Mesh: Intravesical Erosion after Laparoscopic Promontofixation-A Hidden Cost of Durability. J Clin Med Exp Images. 2026: doi: 10.29328/journal.jcmei.1001040; 10: 006-009
-
Obstructive Pyelonephritis Due to Postoperative Ureteral Stricture: A Case ReportAyoub Mamad*,Mohammed Amine Elafari,Mohammed Amine Bibat,Midaoui Moncef,Amine Slaoui,Tarik Karmouni,Abdelatif Koutani,Khalid Elkhader. Obstructive Pyelonephritis Due to Postoperative Ureteral Stricture: A Case Report. J Clin Med Exp Images. 2026: doi: 10.29328/journal.jcmei.1001039; 10: 003-005
-
Could metabolic risk factors contribute to the development of cervical cancer?Maydelín Frontela-Noda*,Eduardo Cabrera-Rode,Maite Hernández-Menéndez,Raquel Duran-Bornot. Could metabolic risk factors contribute to the development of cervical cancer?. Ann Clin Endocrinol Metabol. 2019: doi: 10.29328/journal.acem.1001011; 3: 001-006
Most Viewed
-
Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trialSathit Niramitmahapanya*,Preeyapat Chattieng,Tiersidh Nasomphan,Korbtham Sathirakul. Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trial. Ann Clin Endocrinol Metabol. 2023 doi: 10.29328/journal.acem.1001026; 7: 00-007
-
Physical Performance in the Overweight/Obesity Children Evaluation and RehabilitationCristina Popescu, Mircea-Sebastian Șerbănescu, Gigi Calin*, Magdalena Rodica Trăistaru. Physical Performance in the Overweight/Obesity Children Evaluation and Rehabilitation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001030; 8: 004-012
-
Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging PresentationKarthik Baburaj*, Priya Thottiyil Nair, Abeed Hussain, Vimal MV. Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging Presentation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001029; 8: 001-003
-
Exceptional cancer responders: A zone-to-goDaniel Gandia,Cecilia Suárez*. Exceptional cancer responders: A zone-to-go. Arch Cancer Sci Ther. 2023 doi: 10.29328/journal.acst.1001033; 7: 001-002
-
The benefits of biochemical bone markersSek Aksaranugraha*. The benefits of biochemical bone markers. Int J Bone Marrow Res. 2020 doi: 10.29328/journal.ijbmr.1001013; 3: 027-031

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."