More Information
Submitted: November 20, 2025 | Approved: November 24, 2025 | Published: November 25, 2025
How to cite this article: Amona M, Mavoungou YVM, Ondzotto HN, Kokolo B, Itoua A, Aloumba GA, et al. The Bacteriological Profile of Nosocomial Infections at the Army Central Hospital of Brazzaville. Int J Clin Microbiol Biochem Technol. 2025; 8(1): 009-022. Available from:
https://dx.doi.org/10.29328/journal.ijcmbt.1001032
DOI: 10.29328/journal.ijcmbt.1001032
Copyright License: © 2025 Amona M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The Bacteriological Profile of Nosocomial Infections at the Army Central Hospital of Brazzaville
Medard Amona1*, Yolande Voumbo Matoumona Mavoungou2, Hama Nemet Ondzotto3, Benjamin Kokolo1, Armel Itoua3, Gilius Axel Aloumba2,3 and Pascal Ibata1
1Army Central Hospital of Brazzaville, Republic of the Congo
2University Marien N’GOUABI, Republic of the Congo
3University and Hospital Center of Brazzaville, Republic of the Congo
*Address for Correspondence: Medard Amona, MD., Specialist in Infectious and Tropical Diseases, MPH in Public Health, Option: Program management, Email: [email protected]
Nosocomial infections are infections acquired during a stay in a healthcare facility, representing a major public health challenge worldwide, and particularly in Africa, due to their frequency, potential severity, and associated costs. In Congo, their epidemiological profile is not yet well understood.
It’s in this context that we undertook to conduct a retrospective descriptive study on nosocomial infections between January 1, 2012, and December 31, 2016, in the internal medicine department of the Army Central Hospital of Brazzaville, in order to analyze the bacteriological profile of nosocomial infections.
The study involved 189 patients. The results revealed that hospital-acquired infections were frequent, with a female predominance (71.43%), an average age of 32 years, and risk factors including self-medication with antibiotics (51%) and urinary catheterization (39%). Urinary tract infections were the most common (57%), with Escherichia coli as the main pathogen (17%), and mortality from these infections reached 53%.
The study highlighted a high mortality rate linked to hospital-acquired infections, primarily associated with HIV status and self-medication. Management, prevention, and infection control measures, including improved antibiotic stewardship, are necessary to reduce mortality.
Globally, the quality of healthcare delivery at all levels of the healthcare system is affected by numerous factors, including nosocomial infections, which have become increasingly common in recent decades. [1]. Nosocomial infections are defined as infections that are identified between 48 and 72 hours after a patient is admitted to a hospital [2]. In Africa, their prevalence varies from country to country [3]. They increase the cost of medical care, worsen patient morbidity and mortality, and prolong hospital stays [4]. Healthcare providers are also at risk of contracting these infections and, as a result, adding a functional burden to the healthcare system [5]. The causative agents and their resistance profiles vary according to region and geographical location [6].
In sub-Saharan Africa, in relation to the low socio-economic level, the technical platform that would allow for a definitive diagnosis in record time remains limited and unsatisfactory; hospital infection control committees (CLINs) remain largely ineffective or non-existent in some facilities; and scientific data on these issues are scarce. Knowledge of the bacterial ecology of each healthcare setting and the resistance profile is therefore necessary in order to formulate relevant recommendations on the empirical treatment of infections.
In Congo, this perspective is relevant and deserves our full attention. The objective of this study was to evaluate the bacteriological profile of nosocomial infections and their main characteristics in the internal medicine department of the Army Central Hospital of Brazzaville-Congo.
Type of study, period, and location of study
We conducted a retrospective descriptive study of hospitalized patients from January 1, 2012, to December 31, 2016. It was carried out in the internal medicine department of the central military hospital in Brazzaville.
Patient selection criteria
Inclusion criteria: Patients over 18 years of age with a complete medical record for the parameters studied were included.
Exclusion criteria: Patients who didn’t consent to participate in the study, as well as patients with a nosocomial infection who were not hospitalized in the internal medicine department of the Army Central Hospital.
The study will proceed in two stages:
Recruitment from the medical biology laboratory’s records based on the results of patients who underwent testing involving resistant organisms,
The search for registration numbers in the register of the internal medicine department of the Army Central Hospital of Brazzaville, with the collection of sociodemographic and clinical data in the patient files.
Operational definitions
- A urinary tract infection was defined by the presence of positive quantitative culture ≥ 105 microorganisms/ ml, with a maximum of two microbial species isolated.
- Catheter-related infection is defined by the presence of pathogenic microorganisms on the surface of the catheter.
- Pneumonia is defined by the presence of recent and progressive radiological opacities in the lung parenchyma, purulent sputum, and fever of recent onset with microbiological confirmation of the presence of a microorganism in bronchial secretions.
- Bacteremia is defined by the presence of a pathogenic microorganism in the blood.
- Severe Invasive Conditions (SIs) are defined as any other potentially life-threatening condition associated with nosocomial infection.
Description of laboratory techniques [7]
Urine cytobacteriological exam (CBEU):
Levy: It is important to strictly adhere to the rules of antisepsis, collection, and preservation for a correct interpretation of the cytobacteriological exam of urine (CBEU).
Typically, the first urine should be discarded, and the midstream urine collected in a sterile container (at least 20 ml), taking care not to touch the top edge of the container. The mycobacteria test will be performed on the entire morning urine sample for 3 consecutive days.
In women, the challenge lies in preventing contamination of the urine sample by the commensal flora of the urethra and external genitalia. Therefore, it’s essential to remind the patient of the importance of washing her hands and then carefully cleansing the urethral meatus and vulvar area in a single motion from front to back with mild soap (rinsing thoroughly), followed by a non-foaming antiseptic (gynecological povidone-iodine or aqueous chlorhexidine), moving from the labia minora to the labia majora, avoiding the urethral meatus.
For men, retraction of the foreskin is necessary. In cases of prostatitis, urine is collected from the first stream.
In a probe patient after simple hand washing, the sample can be taken directly by puncturing the catheter: clamp the catheter above the puncture site for 10 minutes (place a gauze pad between the clamp and the catheter to avoid damaging it), disinfect the puncture site with a sterile gauze pad soaked in antiseptic, collect the urine through the puncture site using a monovette or sterile syringe, and transfer the urine into the sterile container. Sampling devices are currently available on catheterization systems.
In an incontinent patient, Intermittent catheterization should be performed after hand washing, and the midstream of the stream should be collected in a sterile bottle, taking care not to bring the bottle into contact with the genital area.
In specific circumstances, the sample may be taken by suprapubic puncture (a specialized procedure) by directly drawing urine from the bladder using a syringe after careful disinfection of the skin.
In all cases, the bottle must be hermetically sealed, identified, and accompanied by a prescription specifying the time of collection, the patient’s temperature, their antibiotic treatment, and any other relevant information.
Urine samples must be transported to the laboratory quickly to prevent microbial growth. Conditions for conservation are as follows: maximum two hours at room temperature or 48 hours if the bottle contains a preservative such as boric acid; maximum 24 hours at 4 °C, knowing that beyond 12 hours, leukocytes begin to deteriorate, which can distort the count.
Biological analyses: Performing a urine culture involves several steps:
• Macroscopic exam of urine;
• Microscopic exam:
_ enumeration of leukocytes and red blood cells(under physiological conditions, urine contains less than 1,000 leukocytes or erythrocytes per ml),
_ Search for crystals, cylinders, and microorganisms by direct examination and by examination of the smear prepared from the centrifuged pellet and stained with Gram stain. The presence of epithelial cells of vaginal origin indicates contamination.
• Their culture, which allows for a quantitative assessment of bacteriuria and an antibiogram.
Results and interpretation
The correct interpretation of the urine culture must take into account numerous parameters (Table 1):
| Table 1: Interpretation of the CBEU | ||||
| Clinical signs | Leukocyturia ≥ 104/ml | Number of species | Bacteriuria | Comment |
| + | + | ≤ 2 | Acute cystitis ≥ 103 CFU/ml if category 1 ≥105 CFU/ml for other bacteria Acute pyelonephritis ≥ 104 CFU/ml Acute prostatitis ≥ 103 CFU/ml Healthcare-associated urinary tract infections. ≥ 103 CFU/ml in the non-catheterized patient. ≥ 105 CFU/ml in the catheterized patient. |
Urinary tract infections |
| + | + | < 103 CFU/ml | Inflammation without bacteriuria: ongoing antibiotic use, slow or difficult-to-culture bacteria, non-infectious etiology. | |
| + | - | ≤ 2 | ≥ 103 CFU/ml | Urinary tract infection (urine culture needs to be repeated) or immunosuppression. |
| - | Variable | ≥ 1 | ≥ 105 CFU/ml | Contamination or colonization. |
| - | - | < 103 CFU/ml | No urinary tract infection | |
| Variable | Variable | ≥ 3 | Probable contamination: repeat urine culture. | |
• The context: particular conditions (immuno-compromised, catheterized patient), existence of prior antibiotic treatment, community-acquired or healthcare-associated infection…;
• The presence of a fever or of urinary symptoms;
• Leukocyturia: a leukocyturia ≥ 104/ml is indicative of an inflammatory process (in catheterized patients, leukocyturia is not informative). The absence of leukocyturia has a good negative predictive value except in immunocompromised patients (neutropenic, transplant recipients) or in cases of early urinary tract infection.
• The nature of the isolated microorganisms: not all have the same level of involvement in the etiology of urinary tract infections (Table 2);
| Table 2: Categorization of microorganisms according to their level of involvement in the etiology of urinary tract infections | |||
| Category 1 | Category 2 | Category 3 | Category 4 |
| Pathogens are systematically responsible for urinary tract infections. | Pathogens involved in nosocomial infections or if there are predisposing anatomical or iatrogenic factors. | Suspicious pathogens | Contaminants |
• The number of microorganisms isolated(mono- or multi-microbial nature of cultures): In practice, beyond two different types of colonies, the analysis suggests contamination, and the sample must be renewed.
• The bacteriuria rate (CFU: Colony Forming Unit):
- Bacteriuria < 103 CFU/ml: absence of infection in the absence of ongoing antibiotic therapy;
- Bacteriuria ≥ 105 CFU/ml: probable infection;
- Between 103 and 104 CFU/ml: zone of uncertainty.
• In community infections:
• In the presence of clinical signs and/or significant leukocyturia, the presence of bacteriuria at 103 CFU/ml is taken into account for acute cystitis if the bacterium belongs to category 1.
• This threshold is higher (105 CFU/ml) for other bacteria, particularly enterococci.
• In pyelonephritis:
• The threshold is fixed at 104 CFU/ml and in prostatitis at 103 CFU/ml.
• In healthcare-associated infections:
• The threshold for bacteriuria is fixed at 103 CFU/ml in the non-catheterized patient and at 105 CFU/ml in the patient with bladder catheterization or other access to the urinary tree.
Note:
The presence of bacteriuria without clinical signs should raise suspicion of colonization.
The presence of clinical signs associated with leukocyturia, even if the culture is negative, should suggest slow-growing or difficult-to-culture bacteria.
The urine culture (CBEU) is a well-defined examination whose two critical stages are:
The withdrawal is too often ≪victim of its apparent simplicity;
The microbiological interpretation must be based on irreproachable decision-making arguments.
Bronchial secretion analysis protocol
Levy: It is done in the morning upon waking, after oral hygiene, and then transported directly to the laboratory within 30 minutes. Do not store.
Macroscopic exam: Macroscopic exam assesses the appearance of sputum, which may be mucous, mucopurulent, or hemoptysis, and the appearance of aspirated fluids, which may be fluid or homogeneous.
Microscopic exam: After fluidization, homogenization:
Coloring with Mary Grunwald, GIEMSA for:
• Pharyngeal cells:
- If cells < 25/ Fields: Good sampling,
- If cells > 25/ Fields: Reject sampling.
• Leukocytes:
- If leukocytes > 25/Fields: Good sampling,
- If leukocytes < 25/ Fields + Monomorphic flora: Good sampling
- If leukocytes < 25/ Fields + Monomorphic flora: Reject the sample.
Gram staining for bronchial cells, alveolar cells, and bacteria
- If monomorphic flora + Neutrophilic Polynuclear cells > 25/ Fields: Cultivate.
- If predominance of one germ + Neutrophils > 25/ Fields: Cultivate.
- Other situations: Do not cultivate.
Culture: Culture is performed on media inoculated with fresh blood agar or cooked blood agar combined with growth factors. Other media are used depending on the Gram stain. The technique employed is a 1/1000 dilution of the samples. The result is the identification of specific pathogens.
Cytobacteriological exam of bronchopulmonary secretions: It is useful for the diagnosis of Pneumonia. The difficulty lies in obtaining a sample with a minimum of contaminants related to the commensal flora of saliva and the oropharynx.
The levy
Expectoration or sputum (ECBC): The collection of sputum must follow a rigorous protocol: it must be done in the morning, upon waking, after rinsing the mouth with sterile distilled water, and during a coughing effort, with the help of physiotherapy if needed.
The sputum is collected in a sterile container. This is a non-invasive and easy-to-perform sample collection, but the risk of contamination by oropharyngeal flora is significant.
Endotracheal suction (ETS/ETA)
Aspiration of secretions via the endotracheal tube is an alternative method when invasive procedures are contraindicated or impossible to perform. The risk of contamination by oropharyngeal flora is significant.
Bronchoalveolar lavage (BAL) and mini-BAL (mini-BAL)
The technique consists of instilling, after blocking the bronchofibroscope, 50 ml samples of physiological serum (37 °C) into a segmental or subsegmental bronchus 4 to 6 times, allowing between 20 and 60% of the injected quantity to be collected.
Bronchoalveolar lavage (BAL) has several advantages: absence of contamination by oropharyngeal flora, alveolar exploration of a larger pulmonary area than percutaneous nasal aspiration (PNA), and collection of a greater quantity of secretions. In intubated and ventilated patients suspected of having nosocomial pneumonia, the concordance between PNA and BAL is approximately 90%.
The mini-LBA or mini-wash involves instilling 20 to 50 ml, but only allows for the collection of 2 to 3 ml of sample.
LBA is particularly useful for the diagnosis of pneumonias observed in immunocompromised patients, allowing the detection of bacteria (Nocardia, mycobacteria, mycobacteria, Mycoplasma pneumoniae, Actinomyces) but also viruses (Cytomegalovirus, Herpes), parasites (Pneumocystis jirovecii, Toxoplasma gondii), fungi and yeasts (Aspergillus, Cryptococcus neoformans, Candida spp).
Gastric intubation
It is reserved for the search for mycobacteria. Performed on an empty stomach, it allows for the collection of tracheal secretions regurgitated during the night.
Biological analyses
Microscopic exam
One of the objectives is to assess contamination by salivary flora.
On direct exam of sputum, the presence of epithelial cells indicates salivary contamination, and that of polymorphonuclear leukocytes indicates infection.
Gram staining allows us to see characteristic morphologies such as those of pneumococci.
In cases of suspected tuberculosis, specific stains (auramine, Ziehl-Neelsen) can be performed on lung samples and gastric lavage.
Culture: It’s performed after a step of liquefying bronchial secretions and dilution to enumerate bacteria present in the sample: the culture is therefore quantitative. It’s necessary to specify the search for certain infectious agents: mycobacteria, lesion-forming bacteria, and fungi grow on specific media; Nocardia and Actinomyces have a slow growth rate (20-day incubation period).
Results and interpretation: Numerous bacteria are part of the commensal flora of the oropharynx. These include coagulase-negative staphylococci and streptococci other than S. pneumoniae, corynebacteria and Neisseria.
Other bacteria, responsible for pleuropulmonary infections, can transiently colonize the upper respiratory tract, in particular Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes and Moraxella (Or Moraxella) catarrhalis.
To differentiate between infection and colonization, three elements must be taken into account:
- Transport and take charge quickly the sample is taken in the laboratory. The goal is to prevent the proliferation of commensal bacteria at the expense of fragile bacteria such as S. pneumoniae;
- Eliminate the samples whose microscopic examination shows obvious salivary contamination (Table 3): If the ECBC or AET is salivary, it will not be cultured and a new sample will be taken. will be redone;
- To proceed with quantitative analysis regarding bacterial flora: the significance threshold depends on the type of sample (Table 4). The presence of a monomorphic flora suggests infection, and the number of identified species should not exceed two.
| Table 3: Interpretation of microscopic exam of the urine culture. | |||
| Classes | Cells per field | Interpretation | |
| Epithelial | Leukocytes | ||
| 1 | > 25 | < 10 | Salivary contamination: do not culture and repeat the sample. |
| 2 | > 25 | 10-25 | |
| 3 | > 25 | > 25 | |
| 4 | 10-25 | > 25 | Acceptable sample: suitable for cultivation. |
| 5 | < 10 | > 25 | |
| Table 4: Significance threshold according to sampling type | |
| Levy | Significance threshold |
| Expectoration | ≥ 107/ml 1 to 2 species only |
| AET | ≥105/ml |
| PDP | ≥103/ml |
| LBA | ≥104/ml |
| Mini-LBA | ≥103/ml |
In case of severe pneumonia it’s preferable to collect pulmonary secretions using invasive but more reliable methods (PDP, LBA).
If the initial bacteriological results are negative, other etiologies (viral, parasitic or fungal) or bacteria with difficult growth should be sought.
In the event of atypical pneumonia, think about Chlamydia psittaci and Chlamydophila pneumoniae, mycobacteria and Mycoplasma pneumoniae.
The diagnosis of bronchopulmonary infections must therefore include a qualitative and quantitative analysis of bronchopulmonary secretions combined with a dialogue with the clinician.
Blood culture protocol: Blood culture is used to diagnose bacteremia (or fungemia). This entity encompasses numerous clinical situations, from simple postprandial bacteremia to severe sepsis.
Levy: Blood culture is the inoculation of blood into a liquid culture medium. Usually, an aerobic bottle and an anaerobic bottle are used.
Sample collection method: The goal is to prevent contamination of the sample. After handwashing and the person collecting the sample wearing a mask and gloves, the sample is taken by venipuncture, starting, depending on the collection system, with either the anaerobic or aerobic collection bottle. Collecting blood through an intravascular device increases the risk of contamination. It’s necessary to disinfect the cap of the blood culture bottles and the puncture site with an alcohol-based antiseptic.
Quantity of blood drawn: The volume of blood drawn determines the sensitivity of the test. In adults, it must be at least 20 ml, or 10 ml per vial. The optimal volume is 40 to 60 ml, requiring a total of 4 to 6 full vials.
Interval between samples: Whether multiple samples are taken (spacing out over time, 2 to 3 samples from 2 vials) or a single sample (taken simultaneously, 1 single sample from 4 to 6 vials), the sensitivity is equivalent. However, the risk of contamination increases with multiple samples, and interpretation becomes more complex.
Single sampling is not recommended for infective endocarditis (collect 3 blood cultures over 24 hours) and infections related to an intravascular device.
Transportation: Blood cultures should be sent to the laboratory as soon as possible.
Biological analyses
Composition of blood culture bottles: In the culture bottles, the blood is diluted in a culture broth (1/5 to 1/10) containing an anticoagulant (sodium polyanethol sulfonate or SPS), which limits the action of inhibitory substances in the blood (lysozyme, complement, phagocytic cells, antibiotics). Some bottles also contain adsorbents (resins, activated charcoal) that limit the bactericidal activity of the blood and the activity of any antibiotics present. There are media designed for use in automated analyzers. In developing countries, it is possible to prepare one’s own media using brain-heart broth supplemented with SPS.
Choice of growing conditions: The vials are incubated in an aerobic and anaerobic atmosphere at approximately 35°C for 7 days (this time can be reduced to 5 days with automated systems, but should be increased to 3 weeks if endocarditis is suspected). Bacterial growth is detected visually or automatically. Automated systems allow for continuous growth detection and are more sensitive and faster.
When resources are lacking, anaerobic culture bottles may not be routinely collected. In such cases, they are only collected in gynecology or digestive surgery departments, or when an anaerobic infection is suspected.
Treatment of positive vials: As soon as a positive vial is detected, Gram staining of the liquid medium allows for rapid guidance of antibiotic therapy. For some bacteria, an antibiogram can be performed immediately. The results will be communicated to the clinician at each stage.
Results and interpretation
Nature of the bacteria identified and clinical significance: Some microorganisms are always pathogenic and do not pose any problems of interpretation. These are: Staphylococcus aureus, Escherichia coli and other enterobacteria, P seudomonas aeruginosa, and Candida.
On the contrary, the Bacillus, Corynebacteria, and Propionibacterium are responsible for bacteremia in less than 5% of cases.
Implicating Streptococcus viridans, Enterococcus, and Coagulase-Negative Staphylococci (CNS) is even more difficult. The majority of CNS isolates are contaminants, and only 10 to 30% of isolates have clinical significance.
Number of positive vials and clinical significance: It is dangerous to consider the number of positive blood culture bottles to assign clinical significance. Indeed, in one-third of cases, contaminants grow in both bottles, and half of the pathogens grow in only one.
Case of polymicrobial blood cultures: These cases affect children (10% of cases) and immunocompromised patients (30% of cases). In both cases, all species present must be considered as having the same infectious potential.
Catheter swabbing protocol
The levy: As a general rule, the swabs have a poor yield: they are not suitable for the survival of desiccation-sensitive bacteria or anaerobic bacteria. Allowing only superficial sampling, they easily collect contaminating flora. If their use is the only alternative, they can be moistened before sampling with sterile saline solution and must be immersed in a transport medium for transport to the laboratory. The most effective samples are those taken with the syringe, the surgical instruments, and the biopsies.
For closed collections, the syringe aspiration (a large-bore needle) is the best way. To preserve the viability of anaerobic bacteria, expel the air from the syringe, remove the needle, and seal with a stopper.
To avoid drying out, when the volume aspirated with the syringe is small, it is possible to aspirate a little sterile physiological saline (100 to 200 μl) secondarily.
In case of skin inflammation, erysipelas or hypodermitis, the site must be disinfected, a little saline solution injected, trying to aspirate as much as possible.
Chronic lesions (ulcers, bedsores) these lesions are always contaminated by skin flora. They are only sampled if accompanied by local or general signs of inflammation (adenitis, fever). In such cases, the wound must be cleaned with saline solution, removing necrotic areas, then the base of the lesion should be aspirated, or ideally, a biopsy should be performed, or the active edge of the lesion should be curetted.
In cases of osteitis, five deep surgical biopsies, well documented regarding their location, will be performed.
For viral searches, in particular the Herpes virus, it is preferable to perform the sampling at the vesicle stage: aspiration of the fluid (less effective because it is low in cells), scraping of the erosive lesion, or application of the vesicle roof to a slide. Tzanck cytology is simple to perform but lacks sensitivity and specificity. Immunofluorescence techniques are practical and more affordable than PCR.
In all cases, clinical information is essential because it will allow the implementation of appropriate means for the search for certain infectious agents (mycobacteria, anaerobes, fungi) as well as for the interpretation of the results by the biologist.
Biological analyses
The laboratory can quickly provide the results of Gram staining: presence of polymorphonuclear leukocytes, presence and morphology of bacteria (interpretation is difficult on contaminated samples, such as superficial wounds). Some fragile and difficult-to-cultivate anaerobes will only be visible upon direct exam.
Results and interpretation
The detection of saprophytic bacteria on the skin is interpreted as contaminants: Coagulase-Negative Staphylococci, Corynebacteria, Propionibacterium, and Bacillus. However, some Coagulase-Negative Staphylococci (such as S. lugdunensis), or Corynebacteria (erythrasma) (C. minutissimum) have virulence factors and must be taken into account.
When anaerobes are responsible for the infection, it is often polymicrobial, and isolating all the anaerobes involved is difficult.
In chronic open infections, even bacteria that are usually pathogenic can be part of a colonizing flora.
Study variables
The variables studied concerned socio-epidemiological aspects, including age and sex; clinico-biological aspects, namely the underlying conditions of the nosocomial infection (HIV, Diabetes, Cancer, others), risk factors (bladder catheterization, catheterization, prior antibiotic therapy), types of nosocomial infections (urinary tract infection, respiratory infection, bacteremia, catheter-related infection), frequently used antibiotics (β-lactams, fluoroquinolones, sulfonamides and others), microbiological analysis, and antibiogram; and finally prognostic aspects, namely hospital mortality related to nosocomial infections and hospital mortality according to the type of nosocomial infection.
Data collection
The data was collected from a pre-established questionnaire.
Ethical considerations and conflict of interest
Ethical approval was obtained from the ethics committee of the Central Military Hospital. Authorization with approval from the Direction Centrale of Health Service to the Congolese Armed Forces was also obtained. Confidentiality and anonymity were guaranteed for all patients. Written consent was provided by each participant. This research received no funding (public, commercial, or for-profit). There are no conflicts of interest.
1. Ethical approval details and consent from participants or their representatives: Ethical approval for the study on nosocomial infections at the Army Central Hospital of Brazzaville was obtained through validation by the hospital’s ethics committee. Informed consent was obtained from patients who agreed to participate or not, after receiving complete information about the study. For patients unable to give consent, authorization was requested from their legal representatives or families.
Ethics Committee: Ethical approval was obtained from the ethics committee of the Army Central Hospital of Brazzaville.
2. Participant consent:
- Informed consent: The volunteer patients gave their informed consent after being informed of the nature of the study.
- Legal representatives: For patients unable to give their consent (for example, due to their state of health), authorization was requested from legal representatives or families.
- Nature of the study: It’s essential to note that the study focuses on nosocomial infections acquired in a hospital setting.
Data entry and analysis
The data were entered using Excel for Windows and analyzed with SPSS 21 software. Statistical analysis of the mean and standard deviation was used for the numerical variables.
Sociodemographic aspects
Age: The average age was 32±11 years, with extremes from 18 to 80 years; the most affected age group was 20 to 40 years.
Sex: Males accounted for 54 cases (28.57%) and females for 135 cases (71.43%). The male-to-female ratio was 0.4
Clinical and biological aspects
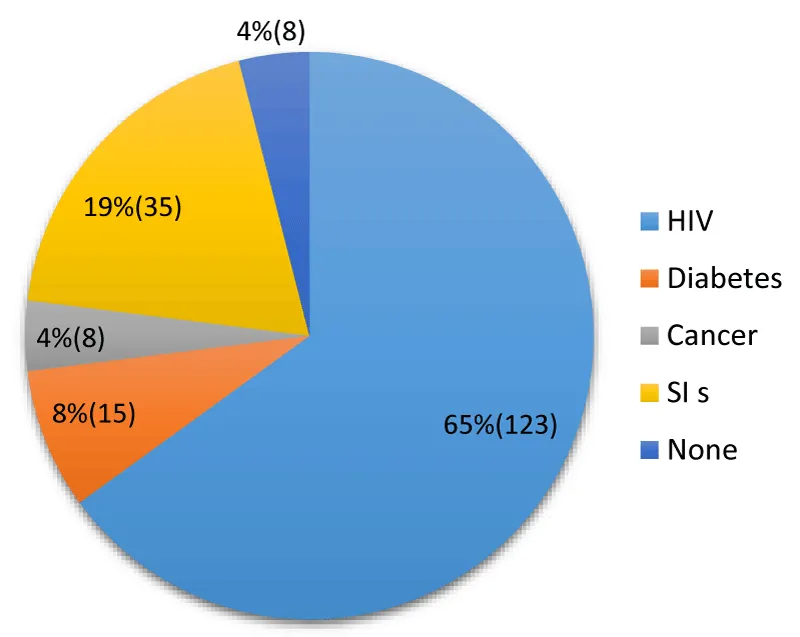
The setting in which nosocomial infection occurs: The rate of immunosuppression in nosocomial infections is 77% (146/189) (Figure 1).
Figure 1: Distribution according to the setting in which nosocomial infection occurred.
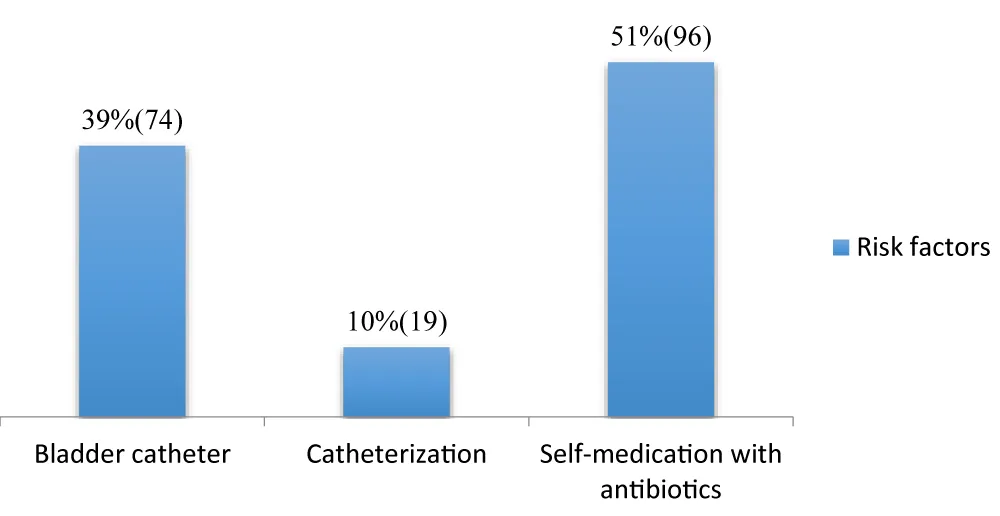
Risk factors: (Figure 2)
Figure 2: Distribution of risk factors.
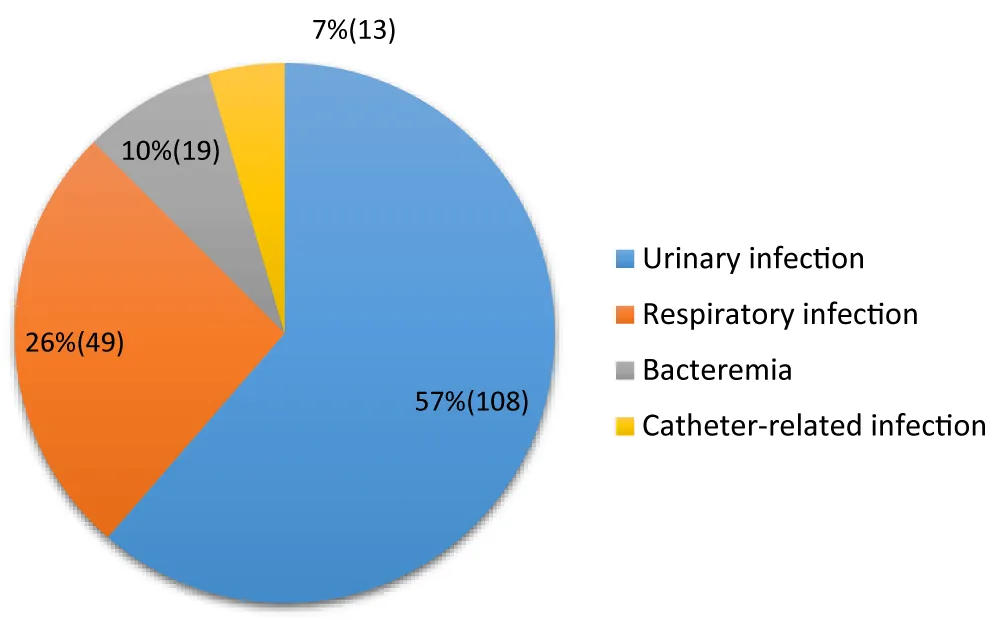
Types of nosocomial infections (Figure 3)
Figure 3: Distribution according to the types of nosocomial infections.
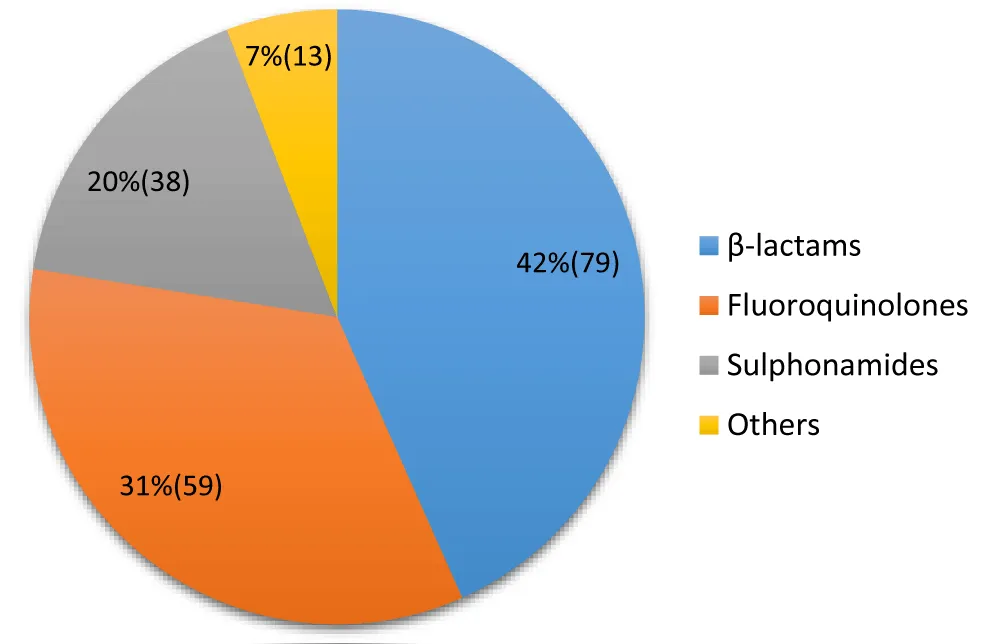
Antibiotics frequently used in self-medication (Figure 4)
Figure 4: Distribution of frequently used antibiotics.
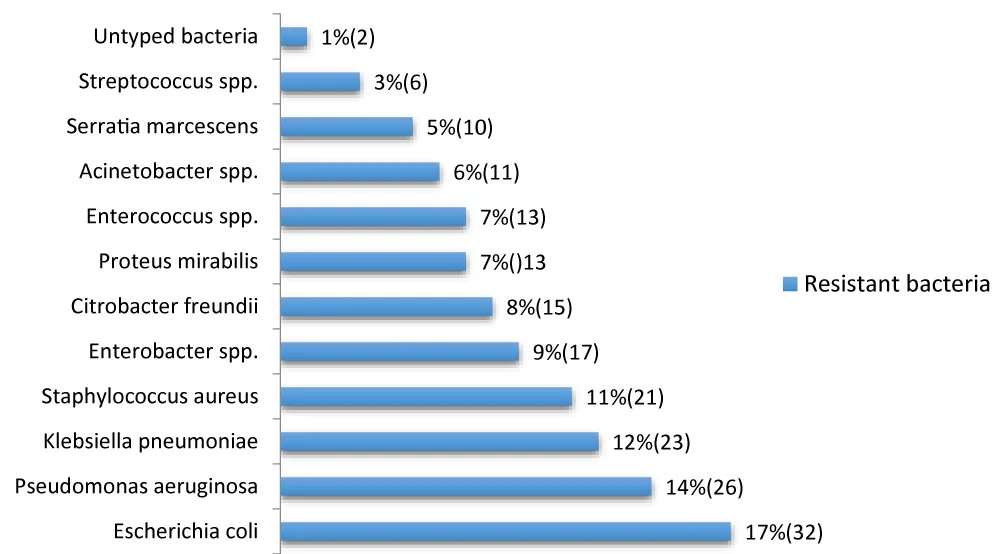
Typology of resistant bacteria (Figure 5)
Figure 5: Distribution of frequently used antibiotics.
Antibiotic resistance profile of bacteria (Table 5)
Prognostic aspects
Hospital mortality related to nosocomial infections: We have recorded 100 cases of death from nosocomial infection, representing a hospital mortality rate linked to nosocomial infection of 53%.
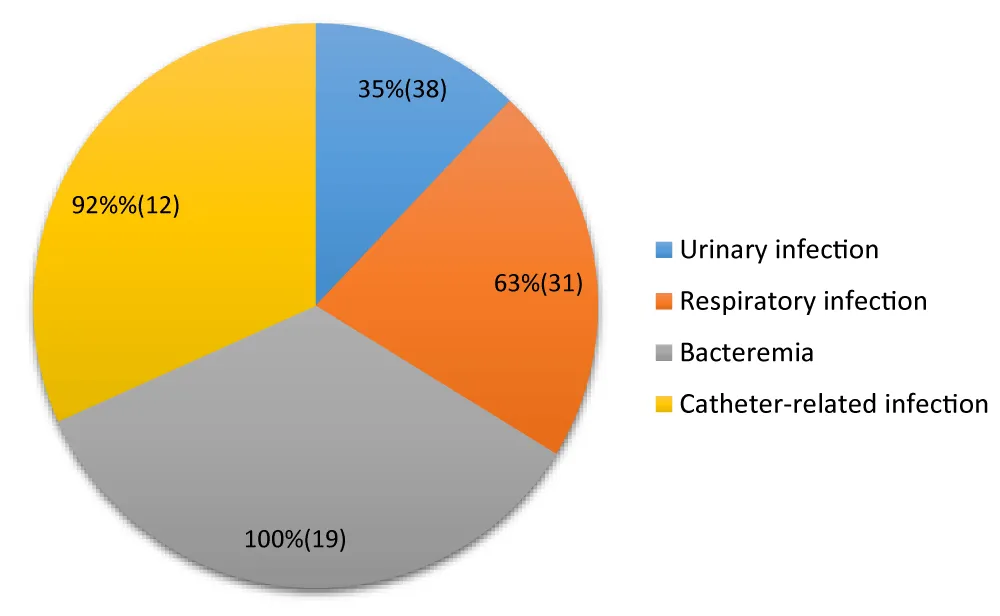
Hospital mortality according to the type of infection (Figure 6).
Figure 6: Distribution of hospital mortality according to the type of nosocomial infections.
Temporal boundaries of the dataset and brief justification for labeling the time frame as pre-pandemic
The author clearly indicates the precise start and end dates of data collection.
The study period was January 1, 2012, to December 31, 2016. These dates define the time limits of the dataset used for the analysis.
The justification for describing this period as “pre-pandemic” is based on the fact that the data collection took place before the emergence and widespread spread of the COVID-19 pandemic, which began in late 2019 and was officially declared a pandemic by the World Health Organization (WHO) in March 2020.
- The data reflect a public health context not impacted by the major upheavals (strengthened hygiene protocols, reorganization of hospital services, increased pressure on resources, etc.) introduced by the global health crisis linked to SARS-CoV-2.
- The chosen period allows for the establishment of a baseline assessment (basic data) of the bacteriological profile of nosocomial infections in the establishment, under "normal" operating conditions, thus providing a basis for future comparison with data collected during or after the pandemic.
- The publication was deemed particularly relevant after the emergence of COVID-19 to serve as a point of comparison (pre-pandemic baseline data) with the post-pandemic situation, yet it was published more than 5 years late.
Sociodemographic characteristics
The average age of 32 is consistent with the demographic structure of Congo, which is characterized by a predominantly young population. The fact that the most affected age group is between 20 and 40 years old suggests that this population, although not systematically the most vulnerable from an immunological point of view (unlike infants or people over 65), is probably the most represented in the medical department of the Brazzaville Central Military Hospital, the hospital services where the risk of nosocomial infections is high.
Our results differ from some studies conducted in developed countries, where advanced age (defined as ≥ 65 years) is a major risk factor for nosocomial infections. However, the results of this study are comparable to those of other work conducted in Lubumbashi in the Democratic Republic of Congo (DRC), which revealed a mean patient age of 37.5 ± 10 years, with the majority of cases between 46 and 60 years, which remains within a relatively young age range compared to Western studies [8]. At the University Hospital Center (CHU) of Brazzaville, the pulmonology department also reports a relatively young average age (around 50.6 years with a peak between 35 and 65 years), which is consistent with the local demographic profile [9].
The study reveals a marked predominance of females (71.43%) compared to males (28.57%) among cases of nosocomial infections, with a male-to-female (M/F) ratio of 0.4. This observation is consistent with data from other studies conducted in Congo [10]. A study on the prevalence and contributing factors of nosocomial infections in Douala reported a similar distribution with 60.3% women versus 39.7% men [11]. The male-to-female sex ratio of 0.4 (approximately 1 man for every 2.5 women) is lower than the sex ratio of 1:2 observed in another African study [12], which suggests an even greater representation of women in our series.
Site of occurrence (comorbidities)
The fact that 65% of nosocomial infections occur in HIV-infected patients is striking and constitutes a major epidemiological characteristic in the Brazzaville context. This figure is significantly higher than HIV prevalence rates in the general population or among hospitalized patients in many developed countries, but it is consistent with the high prevalence of HIV/AIDS in sub-Saharan Africa. HIV is a well-known immunosuppressive factor that compromises the host’s immune defenses, thereby increasing susceptibility to opportunistic and nosocomial infections [13].
The high prevalence reported here suggests that the internal medicine department of the Brazzaville Central Military Hospital is managing a significant burden of immunocompromised patients, which has significant implications for infection control measures.
Studies conducted in the DRC have also highlighted the prevalence of risk factors, although such high specific figures for HIV vary depending on the studies and hospital settings [14; 15].
The fact that 77% of patients with nosocomial infections are immunocompromised (HIV, diabetes, cancer, AIS) confirms the crucial importance of underlying conditions in the occurrence of nosocomial infections in this hospital. Diabetic (8%) and cancer (4%) patients are also populations at increased risk of infection due to metabolic disturbances, neutropenia related to chemotherapy, or immunodeficiency related to the disease itself. [16; 17]. This high rate of immunosuppression underscores that nosocomial infections in Brazzaville are not solely linked to lapses in hygiene measures but are also heavily influenced by the intrinsic vulnerability of hospitalized patients. This could partly explain certain aspects of the local bacteriological profile, as immunocompromised patients may be infected by opportunistic or atypical pathogens in addition to classic nosocomial pathogens.
These results differ from the WHO’s overall prevalence data in other regions, such as the Mediterranean region, where classic risk factors such as the use of invasive devices (urinary catheters, central catheters, mechanical ventilation) are often predominant [18]. Although these devices are also used in Brazzaville, immunosuppression appears to be the most significant individual risk factor in this series. The 4% proportion of patients with no identified cause of nosocomial infections could be due to environmental or healthcare-related factors not recorded in the study, or simply to the proportion of infections that occur independently of underlying health conditions.
Thus, these data specific to Brazzaville highlight the need for enhanced care for immunocompromised patients, with increased vigilance regarding hygiene measures and the prevention of nosocomial infections adapted to this vulnerable population. Further research is needed to specifically link these immunocompromised conditions to the local bacteriological profile to refine treatment protocols.
Risk factors for the occurrence of nosocomial infections
The data on risk factors in this series (urinary catheter: 39%, catheterization: 10%, self-medicated antibiotic therapy: 51%) highlight the predominant role of invasive procedures and inappropriate antibiotic therapy practices in the context of Brazzaville. These results highlight trends specific to the healthcare environment in Brazzaville, which are both consistent with international scientific literature and reveal local challenges.
Bacterial colonization is almost inevitable with urinary catheterization, and it increases by 3 to 10% per day of catheterization. The high rate observed in Brazzaville (39%) confirms that adherence to aseptic catheter insertion and maintenance protocols is a major challenge in the city’s hospitals. Studies conducted in other African countries, such as the DRC, also confirm the prevalence of urinary tract infections in the local nosocomial landscape [14; 15].
Catheterization (10%) is also a known risk factor for nosocomial infections, primarily catheter-related bacteremia. Although this figure is lower than that of urinary catheters, it remains significant. The risk is directly related to the duration of catheter insertion and adherence to hygiene protocols during catheter handling [19].
The most striking finding, specific to the socio-health context, is that 51% of the bacteriological profile of nosocomial infections is influenced by prior self-medicated antibiotic therapy. This high percentage is alarming and highlights a common, high-risk practice among the population of Brazzaville: self-medication with antibiotics. This practice promotes the selection and spread of multidrug-resistant bacteria, making subsequent nosocomial infections more difficult to treat. The inappropriate use of antibiotics is internationally recognized as the main driver of antimicrobial resistance [20].
In the African context, including in the Congo, easy access to over-the-counter antibiotics and a lack of awareness contribute to this phenomenon. The fact that more than half of the bacteriological profile of nosocomial infections is affected suggests considerable community microbial selection pressure that directly impacts the hospital environment. This may explain the high prevalence of certain resistant bacteria in Brazzaville hospitals, a challenge already documented in similar studies in Africa [21; 22].
The results of this study confirm that invasive devices are major risk factors for nosocomial infections. However, the predominant influence of self-administered antibiotic therapy on the bacteriological profile highlights an urgent public health problem.
It is imperative to strengthen hygiene and care protocols related to the insertion and maintenance of bladder catheters and urinary catheters in health facilities in Brazzaville, to combat antibiotic self-medication at the community level through awareness campaigns and strict regulation of the sale of antibiotics without a prescription in Congo, and finally to conduct continuous microbiological studies to monitor the evolution of bacterial resistance in local hospitals to adapt empirical antibiotic therapy protocols.
Antibiotics are frequently used in self-medication
The study results show that the most frequently used classes of antibiotics for self-medication are beta-lactams (42%), followed by fluoroquinolones (31%) and sulfonamides (20%). Other classes represent a minority share (7%).
Beta-lactams, including penicillins and cephalosporins, are the most prescribed and consumed antibiotics worldwide, including through self-medication. Their accessibility, often without a prescription in informal channels, likely explains this prevalence (42%). However, the inappropriate and incomplete use of these antibiotics promotes strong selection pressure, leading to high levels of bacterial resistance, as shown by various African studies where resistance to ampicillin, a common beta-lactam, is very high [22; 23].
Self-medication with fluoroquinolones (31%) is particularly concerning. These broad-spectrum antibiotics are generally reserved for treating serious infections in hospital settings or when other antibiotics have failed, due to their potentially severe side effects. Their use for self-medication, often for mild or viral infections (for which they are ineffective), directly contributes to the selection of resistant strains, rendering these crucial drugs useless for patients who truly need them. Studies conducted in Brazzaville have already highlighted the problem of inappropriate prescribing, and self-medication exacerbates this phenomenon [24].
Sulfonamides (20%) are an older class of antibiotics, often used in combination (Cotrimoxazole). Significant resistance to this class is frequently observed, particularly due to their historical and widespread use in combating opportunistic infections in HIV/AIDS [25]. The link between community self-medication and resistance profiles in nosocomial infections is crucial. Bacteria circulating in the community are exposed to these antibiotics and develop resistance mechanisms. These resistant bacteria can then end up in hospitals, complicating the management of nosocomial infections where the choice of first-line antibiotics is already limited [26]. The profile of bacterial resistance in Brazzaville is probably influenced by these self-medication practices.
In Congo, the problem of self-medication is known; however, local studies of the frequency of this practice and the lack of knowledge of its consequences among consumers are necessary.
On the typology of resistant bacteria
The distribution of resistant bacteria in our study shows a predominance of Gram-negative bacilli (GNB), which represent the majority of the identified isolates (E. coli, P. aeruginosa, K. pneumoniae, Enterobacter spp., C. freundii, P. mirabilis, Acinetobacter spp., S. marcescens). This finding is consistent with the general scientific literature, which often reports GNB as the main nosocomial pathogens, particularly in urinary tract infections and pneumonia [27].
Escherichia coli (17%) is the most frequent germ in our results. This is consistent with global and African studies, where E. coli is often the microorganism most implicated in nosocomial infections, particularly urinary tract infections. A study conducted in the pulmonology department of the Brazzaville University Hospital also identified E. coli as the most frequent germ (11.9%) [28].
Klebsiella pneumoniae (12%) and Enterobacter spp. (9%) are also present. These bacteria, along with E. coli and Citrobacter freundii (8%), are part of the KES (Klebsiella, Enterobacter, Serratia) and PPM (Proteus, Providencia, Morganella) group, known for their ability to develop resistance mechanisms, including the production of extended-spectrum beta-lactamases (ESBLs).
P seudomonas aeruginosa (14%) is the second most common bacterium. This opportunistic pathogen is a major player in healthcare-associated infections, particularly in intensive care units and among burn patients, due to its intrinsic resistance to many antibiotics.
Acinetobacter spp. (6%) It is also a non-fermentative Gram-negative bacterium whose emergence is worrying, often associated with nosocomial epidemics and known for its multi-resistance.
Staphylococcus aureus (11%) is a notable Gram-positive pathogen. Its presence, although lower than that of Gram-negative bacilli in our overall results, remains significant. The main problem with S. aureus is the existence of methicillin-resistant strains (MRSA), which considerably complicate treatment. A study in Brazzaville reported 24% MRSA among the isolated bacteria, highlighting the extent of this problem locally [29].
Enterococcus spp.(7%) and Streptococcus spp. (3%) are also present, often involved in bacteremia and urinary tract infections.
The typology observed in Brazzaville reflects an epidemiological landscape dominated by resistant Gram-negative bacilli, with a significant proportion of Gram-positive cocci. Comparison with other studies conducted in Congo or in similar contexts shows consistency in the main pathogens involved (E. coli, P. aeruginosa, K. pneumoniae, S. aureus).
These results highlight the importance of establishing continuous and standardized epidemiological surveillance of antimicrobial resistance in Brazzaville health facilities, strengthening hygiene and infection prevention measures, such as hand hygiene and proper sterilization of equipment, to limit cross-transmission of MDROs, and developing local empirical antibiotic therapy protocols, based on the resistance profile of these bacteria, to optimize patient care and preserve the effectiveness of available antibiotics.
The bacterial typology of nosocomial infections in Brazzaville is dominated by Gram-negative bacilli (E. coli, P. aeruginosa, K. pneumoniae), followed by S. aureus. These results, consistent with regional scientific data, confirm the urgent need for targeted actions to combat antibiotic resistance in Congo [30,31].
In-depth studies on specific resistance mechanisms (ESBL, MRSA, etc.) and local risk factors are needed to guide public health interventions.
The results of this study paint a worrying picture of antimicrobial resistance (AMR) in nosocomial infections in Brazzaville. It is alarming and highlights the presence of multidrug resistance (MDR) in the majority of identified strains.
High resistance to third-generation cephalosporins (CTX) and fluoroquinolones (CIP) is noted for E. coli and K. pneumoniae. This strongly suggests a high prevalence of extended-spectrum beta-lactamase (ESBL)-producing bacteria, a major public health problem. ESBL resistance significantly limits treatment options [32].
Notably, imipenem (IPM), a carbapenem, maintains excellent sensitivity against most Gram-negative bacilli (E. coli, P. aeruginosa, K. pneumoniae, Enterobacter spp., Citrobacter freundii, Serratia marcescens, Acinetobacter spp.). Imipenem thus appears to be a cornerstone in the treatment of these severe nosocomial infections in Brazzaville. However, the pressure on carbapenem use must be managed carefully to avoid the emergence of carbapenemases, the dissemination of which would be a therapeutic disaster.
P seudomonas aeruginosa exhibits high resistance to ciprofloxacin (R), but remains susceptible to imipenem and amikacin (AN). Management of P seudomonas infections is often challenging and relies on the judicious use of antibiotic combinations to prevent the development of resistance during treatment [33].
Staphylococcus aureus shows intermediate (I) or resistant (R) susceptibility to many antibiotics, including penicillin (P), oxacillin (FOX), and ciprofloxacin (CIP). Methicillin resistance, characteristic of MRSA (Methicillin-Resistant Staphylococcus aureus), is likely given the FOX resistance. Vancomycin (VA), although not tested or applicable in the table provided for this profile, is generally the treatment of choice for MRSA [34]. The results show that S. aureus remains sensitive to tetracycline (TE), which could offer a therapeutic alternative in some cases.
Enterococcus spp.This bacterium exhibits a high resistance profile inherent to this genus, with natural resistance to cephalosporins and often to aminoglycosides. Vancomycin resistance, if present, would indicate Vancomycin-Resistant Enterococci (VRE), a major concern in hospital settings.
These data underline the urgent need to strengthen antimicrobial stewardship policies and infection prevention and control (IPC) measures in health facilities in Brazzaville.
The high resistance profile to first-line antibiotics makes the use of carbapenems crucial, although their availability and cost can be challenges in the Congolese context. Furthermore, the lack of precise data on resistance mechanisms (ESBLs, carbapenemases) limits therapeutic optimization.
It is imperative to implement continuous epidemiological surveillance of AMR at the national level, as recommended by the World Health Organization (WHO) and the National Action Plan for Combating Antimicrobial Resistance of the Republic of the Congo [35,36].
The microbiological landscape of nosocomial infections in Brazzaville is dominated by multidrug-resistant Gram-negative bacilli, posing a major challenge to clinical management. The rational use of carbapenems, which remain effective, and the strengthening of hygiene measures are essential to contain this public health threat.
The antibiotic resistance profile of bacteria
Results of bacterial resistance to antibiotics: The overall mortality rate of 53% reported in this study is exceptionally high. In developed countries, mortality attributable to nosocomial infections varies considerably depending on the type of infection and the department (e.g., 7% to 30% for pneumonia, 16% to 35% for bacteremia) [37]. Studies conducted in other African contexts, including the DRC, mention nosocomial infection prevalence rates of around 10.5%, but precise data on directly attributable mortality are often fragmentary [15]. The figure of 53% suggests a particularly heavy burden of morbidity and mortality, potentially linked to diagnostic delays, antibiotic resistance, or suboptimal management, and finally to the influences of comorbidities.
A 100% case fatality rate for bacteremia is alarming. Bacteremia, or septicemia, is a serious complication of nosocomial infections and is associated with significant mortality, generally ranging between 16% and 35% in the scientific literature [38]. A 100% rate in this specific context highlights a critical situation, probably due to the virulence of the germs involved (such as Staphylococcus aureus or P seudomonas aeruginosa, often found in hospital settings), or to the lack of effective and rapid treatment protocols. Studies in Brazzaville have already explored the clinical and evolutionary aspects of sepsis, confirming the severity of these pathologies [39].
The catheter-related infection mortality rate of 92% is also high. Catheter-related infections are frequent, especially in intensive care units, and their mortality rate is significant. This finding underscores the urgent need to strengthen catheter hygiene and maintenance protocols, which are essential for preventing hospital-acquired infections.
Pneumonia is known to be among the deadliest nosocomial infections, particularly in mechanically ventilated patients in intensive care. The 63% rate is high, but close to the upper ranges (up to 30% or more in intensive care) reported in other studies [40,41].
Nosocomial urinary tract infections are the most frequent, but their mortality rate is often lower when properly managed. The 35% rate is high compared to general data. This may reflect the severity of the cases included in the study, significant comorbidities in the patients, or a high proportion of multidrug-resistant organisms. Comparing these results with existing local epidemiological data is essential to guide antibiogram and empirical antibiotic therapy policies.
These results highlight a particular epidemiological situation at the Armed Central Hospital of Brazzaville. However, the site of occurrence (comorbidities) could influence the high mortality rates associated with nosocomial infections.
Influence of the site of occurrence (comorbidities) on mortality: Patients infected by HIV are notoriously immunocompromised and at increased risk of nosocomial infections. A CD4 count < 200/mm³ is a significant risk factor for mortality. The fact that 65% of the study patients were HIV-positive is a key factor in the high mortality observed, as their weakened immune systems struggle to effectively fight the infection. Local studies have already explored the causes of death among patients living with HIV at the University and Hospital Center of Brazzaville, confirming their vulnerability [42,43].
Diabetes is a recognized risk factor for infections in general and nosocomial infections in particular, as it impairs immune function and wound healing [44].
Severe Invasive Conditions (19%) and cancer (4%) often involve invasive procedures (catheters, surgery) and an altered general condition or immunosuppression, increasing the risk of sepsis and fatal complications [45].
Hospital mortality from nosocomial infections in Brazzaville is a serious problem, exacerbated by the high prevalence of severe comorbidities, particularly HIV. Mortality rates, especially for bacteremia and respiratory infections, are high compared to international standards. It should be noted that correlating comorbidities with causes of death using Pearson correlation rules would have revealed the influence with greater specificity.
This cross-sectional study conducted in Brazzaville highlights a major public health issue: high hospital mortality due to nosocomial infections. The overall hospital mortality rate associated with nosocomial infections reaches a critical level of 53%. The case fatality rate varies considerably depending on the type of infection, with particularly alarming mortality for bacteremia (100%) and catheter-related infections (92%), followed by respiratory (63%) and urinary tract (35%) infections.
The epidemiological profile of the deceased patients reveals a high prevalence of severe underlying comorbidities: HIV (65%) is the most frequent condition, followed by severe invasive diseases (19%), diabetes (15%) and cancer (4%).
The overall mortality rate of 53% is higher than the averages observed in some studies, even in an African context, and underscores the severity of the local situation. The 100% mortality rate for bacteremia is particularly concerning, potentially suggesting high resistance to available anti-infective treatments or delayed diagnoses.
The predominance of HIV as a major risk factor for death (65% of cases) is consistent with other studies conducted in Africa, which demonstrate that people living with HIV are more vulnerable to severe infections and increased mortality. The association with other chronic conditions such as diabetes and cancer also exacerbates this risk.
These results raise questions about the effectiveness of infection prevention and control (IPC) measures in Brazzaville health facilities and about the specific care of immunocompromised patients or those with comorbidities.
Therefore, it is imperative to ensure an urgent response by:
- Strengthening programs for the prevention and control of nosocomial infections, which should help to establish nosocomial infection control committees in all health facilities, with particular attention to hand hygiene and care related to catheters and urinary probes.
- The implementation of standardized management protocols for nosocomial infections, taking into account the local bacteriological profile and antibiotic resistance.
- Better integration of the management of comorbidities, particularly HIV, into overall hospital care strategies.
These measures are essential to reduce avoidable hospital mortality at the Army Central Hospital of Brazzaville and improve patient safety.
- World Health Organization. Global Report on Infection Prevention and Control 2024. Geneva: World Health Organization; 2024.
- Carlet J. Healthcare-associated infections [Online]. Paris: High Council for Public Health (HCSP); 2002 [accessed 18 Sept. 2025]. Available from: https://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=ad382323.pdf.
- World Health Organization (WHO). Prevalence of nosocomial infections in 27 hospitals in 12 African countries: results of the WHO point prevalence survey, 2010. Bulletin of the World Health Organization. 2010; 88 (10): 1070-8. Available from: https://pubmed.ncbi.nlm.nih.gov/21226344/
- Durand-Zaleski I, Chaix C, Brun-Buisson C. The cost of healthcare-associated infections. Adsp. 2002; 38: 29-31.
- World Health Organization (WHO). Nosocomial infections: A major challenge for patient safety. World Health Organization Bulletin. 2021; 99 (11): 821-829.
- World Health Organization. WHO guidelines on hand hygiene in health care [Online]. Geneva: World Health Organization; 2009 [accessed September 18, 2025]. 270 p. Available from: https://www.who.int/publications/i/item/9789241597906.
- College of University Professors of Infectious and Tropical Diseases (CMIT), publisher. ePILLY Trop 2022: Tropical Infectious Diseases [Online]. 2022 edition. Lyon: Alinéa Plus; 2022 [accessed 18 Sept 2025]. Available from: https://www.infectiologie.com/UserFiles/File/formation/epilly-trop/livre-epillytrop2022.pdf.
- Kankudja M, Tshibangu D. Epidemiological characteristics of patients in Lubumbashi. Rev Med Congo. 202; 45 (2):110-5.
- Bemba E, Koumeka PP, Ossale-Abacka KB, et al. Profile of respiratory diseases in the elderly in the pulmonology department of the Brazzaville University Hospital. Rev Mal Respir. 2018; 35 (4): 421-428.
- Moyen JM, Lobe I, Ndzeingou S, Koukouikila-Koussounda F. Study of the prevalence of nosocomial infections and risk factors at the University Hospital Center of Brazzaville, Republic of Congo. Pan Afr Med J. 2016; 24: 275. Available from: https://doi.org/10.11604/pamj.2016.24.275.7626
- Nguefack T, et al. Study of the prevalence of nosocomial infections and contributing factors in a hospital in the city of Douala. Pan African Medical Journal. 2016; 24: 275.
- Ouedraogo KM, Traore Y, Kologo JP, Ouattara A, Nikiema T, Ouattara S, et al. Study of the prevalence of nosocomial infections and associated risk factors at Yalgado Ouédraogo Hospital in Burkina Faso. Pan Afr Med J. 2016; 24: 275.
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Updated World AIDS Report 2022. Geneva: UNAIDS; 2022. 256 p.
- Kasongo Kakupa D, Kalenga Muenze P, Byl B, Dramaix Wilmet M. Prevalence and factors contributing to nosocomial infections in the city of Goma in the Democratic Republic of Congo: case of two referral hospitals CBCA-Virunga and Charité maternelle. J Med Public Health Policy Rep. 2024; 3 (2): 66-74.
- Kakupa DK, Muenze PK, Byl B, Wilmet MD. Study of the prevalence of nosocomial infections and associated factors in the two university hospitals of Lubumbashi, Democratic Republic of Congo: case of the University Clinics of Lubumbashi. Pan Afr Med J. 2016 Jul 27; 24 (275): 1-6. Available from: https://doi.org/10.11604/pamj.2016.24.275.7626
- Mohamedi N, Batteux F, Larger E. Does diabetes really impair the immune system? Rev Med Interne 2019 Mar; 40 (3): 179-183.
- Vidal L, Ben dor I, Paul M, Eliakim-Raz N, Pokroy E, Soares-Weiser K, Leibovici L. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No.: CD003992. Available from: 10.1002/14651858.CD003992.pub3.
- WHO Eastern Mediterranean Region. Study provides prevalence data and risk factors for nosocomial infections in hospitals within that region, noting the prevalence of risk factors like invasive devices. East Mediterranean Health J. 2010; 16 (10): 1070-8.
- Lorente L. Prevention of catheter-related infections: which catheter, which route and which insertion technique to choose? Intensive Care Medicine. 2012; 22 (S2):409-416.
- World Health Organization. Antimicrobial resistance: key benchmarks [Online]. Geneva: WHO; 2021 Nov 17 [cited 2025 Sep 13]. Available from: https://www.who.int/fr/news-room/fact-sheets/detail/antimicrobial-resistance.
- Ngoma A, Biyoya L. Antibiotic resistance in Brazzaville hospitals: prevalence and challenges. J Afr Health Sci. 2023; 45 (2): 112-9.
- Da L, Somé D, Yehouenou C, Somé C, Zoungrana J, Ouédraogo AS, Lienhardt C, Poda A. Current state of antibiotic resistance in sub-Saharan Africa. Médecine et Maladies Infectieuses. 2023; 2 (1): 3-12.
- Lebrun S, Sonda T, Dagnra C, Ngandjio A, Abotsi R, Akindele O, et al. Antimicrobial resistance in Africa: a systematic review. J Antimicrob Chemother. 2017 Sep 11; 72 (10): 2757-69.
- Saade D, Jabagi MJ, Bertrand M, Hider-Mlynarz K, Grimaldi L, Zureik M. Use of systemic fluoroquinolones in France between 2014 and 2023. Final report. EPI-PHARE; 2025 Jan 28.
- World Health Organization. Management of infectious complications associated with HIV infection - Recommendations. Geneva: World Health Organization; 2022.
- Krir A, Dhraief S, Messadi AA, Thabet L. Bacteriological profile and antibiotic resistance of bacteria isolated in a burn intensive care unit over seven years. Ann Burns Fire Disasters. 2019; 32 (3): 197-202. Available from: https://pubmed.ncbi.nlm.nih.gov/32313533/
- Dupont A, Martin B. Epidemiology of nosocomial infections: prevalence of Gram-negative bacilli. J Hosp Infect. 2023; 112 (5): 234-45.
- Bopaka RG. Nosocomial infection in the pulmonology department of the Brazzaville University Hospital. Rev Mal Respir Actual. 2021 Jan 10; 13 (1): 164.
- Ndalla AV, Moyen R, Obengui PK, et al. Antibiotic susceptibility of Staphylococcus aureus strains isolated from wounds in Brazzaville (Congo). Pan Afr Med J. 2014; 18 (276).
- Otiobanda GF, Moyen E, Bomelefa-Bompay X, et al. Bacterial ecology of nosocomial infection in the intensive care unit of the Talangai District Hospital in Brazzaville. Pan Afr Med J. 2013 Apr 9; 14: 140. Available from: https://doi.org/10.11604/pamj.2013.14.140.1818
- Dahier F, Bomele RP, Mokondjimobe M, Nsonde-Offoumou RO, Moyen E. Study of the prevalence of nosocomial infections and associated factors in the two university hospitals of Lubumbashi, Democratic Republic of Congo: case of the University Clinics of Lubumbashi. Pan Afr Med J. 2016 Jul 27; 24: 275.
- World Health Organization. WHO warns of widespread resistance to common antibiotics worldwide [Online]. Geneva: World Health Organization; 2025 Oct 13 [cited 2025 Nov 13]. Available from: https://www.who.int/fr/news/item/13-10-2025-who-warns-of-widespread-resistance-to-common-antibiotics-worldwide.
- Trautmann M, Halder S, Hoegel J, Royer H, Haller M. Point-of-use water filtration reduces endemic P seudomonas aeruginosa infections on a surgical intensive care unit. Am J Infect Control. 2008 Aug; 36 (6): 421-9.
- Liu C, Bayer A, Cosgrove SE, Daum RK, Fridkin SS, Gorwitz RJ, et al. Clinical practice guidelines for the management of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011 Feb; 52 (3): 18-55.Available from: https://doi.org/10.1093/cid/ciq146
- Global Antimicrobial Resistance Surveillance System (GLASS): Implementation Manual [Online]. Geneva: World Health Organization; 2015 [cited 13 Nov 2025]. Available from: https://www.who.int/fr/publications/i/item/9789241549400.
- Government of the Republic of Congo, Ministry of Public Health and Population. National Action Plan to Combat Antimicrobial Resistance in the Republic of Congo 2022. Brazzaville: Ministry of Public Health and Population; 2022.
- Pérez A, Dupont B. Mortality attributable to nosocomial infections in developed countries. Rev Infect Control. 2021; 45 (3): 112-9.
- Bouza E, Pérez MJ, Muñoz P, et al. Nosocomial bacteremia: clinical and microbiological epidemiology in a tertiary hospital over a 15-year period (1991-2005). Enferm Infecc Microbiol Clin. 2007 Feb; 25 (2): 95-101.
- Niengo Outsouta G, Monkessa CM, Elombila M, Gallou Leyono-Mawandza PD, Ontsira Ngoyi EN, Tsouassa Wa Ngono GB, et al. Sepsis and septic shock in intensive care in Brazzaville (Congo). Health Sci Dis [Online]. 2022 Dec [cited 2025 Nov 13]; 24 (1). Available from: https://doi.org/10.5281/hsd.v24i1.4135.
- Ossou-Ngui M, Ondzotto G, Moyen G, et al. Prognostic factors of ventilator-associated pneumonia in the intensive care unit at the Brazzaville University Hospital. Bull Soc Pathol Exot. 2010; 103 (4): 265-270.
- Ngassa L, Beka T. Mortality rate of nosocomial infections in intensive care in a hospital in Yaoundé. Pan Afr Med J 2018; 31 (1): 25-30.
- Ossibi Ibara BR, Obengui, Mbemba, Ahoui AC, Ontsira NG, Sekangué Obili G, Elenga Mbolla, Boumandouki P, Puruehnce MF. Causes of death among patients living with HIV in the Infectious Diseases Department of the University Hospital Center of Brazzaville. [Online]. 2014 [accessed 15 Oct 2025]; 7 (2): 5 pages. Available from: https://juniperpublishers.com/arr/pdf/ARR.MS.ID.555630.pdf.
- Ossibi Ibara BR. Neuromeningeal disorders during HIV in the infectious diseases department of the Brazzaville University Hospital: prevalence and factors associated with death. European Scientific Journal [Online]. 2016 [accessed 15 Sept 2025]; 12 (33): 177-88. Available from: https://eujournal.org/index.php/esj/article/view/8326.
- Habonimana A, Gaturagi C, Nsabiyumva F, Iradukunda A. Diabetes and infectious complications: a study conducted at the Kamenge University Hospital Center on 36 cases. Med Afr Noire. 2021; 4 (9).
- MSD Manual. Septicemia and Infectious Shock [Online]. Merck & Co., Inc.; 2025 [accessed September 15, 2025]. Available from: https://www.msdmanuals.com/fr/professional/r%C3%A9animation/sepsis-et-choc-septique/sepsis-et-choc-septique.