More Information
Submitted: 30 September 2020 | Approved: 12 October 2020 | Published: 13 October 2020
How to cite this article: Mbula MMK, Situakibanza HNT, Mananga GL, Mbenza BL, Makulo JRR, et al. Atherogenic risk assessment of naive HIV-infected patients attending Infectious Diseases Service of Kinshasa University Teaching Hospital, Democratic Republic of the Congo (DRC). Int J Clin Microbiol Biochem Technol. 2020; 3: 040-048.
DOI: 10.29328/journal.ijcmbt.1001015
Copyright License: © 2020 Mbula MMK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: HIV; Cardiovascular risks Assessment; Lipid ratios; Kinshasa; DRCongo
Atherogenic risk assessment of naive HIV-infected patients attending Infectious Diseases Service of Kinshasa University Teaching Hospital, Democratic Republic of the Congo (DRC)
MMK Mbula1, HNT Situakibanza1, GL Mananga2, B Longo Mbenza3, JRR Makulo4, MM Longokolo1, MN Mandina1, NN Mayasi1, MM Mbula5, B Bepouka1, GL Mvumbi6, EN Amaela7, DN Tshilumba8, O Odio1, BM Ekila1, A Nkodila1 and BT Buasa9
1Infectious Diseases Service, Kinshasa University Teaching Hospital, Democratic Republic of the Congo
2Neuro Psycho Pathological Center (CNPP), Kinshasa University Teaching Hospital, Democratic Republic of the Congo
3Cardiology Service, Kinshasa University Teaching Hospital, Democratic Republic of the Congo
4Nephrology Service, Kinshasa University Teaching Hospital, Democratic Republic of the Congo
5General Practitioner not yet Assigned, Kinshasa, Democratic Republic of the Congo
6Department of Basic Sciences, Faculty of Medicine, Kinshasa University Teaching Hospital, Democratic Republic of the Congo
7Kinshasa General Military Reference Hospital, Camp Kokolo, Democratic Republic of the Congo
8Higher Institute of Medical Techniques, Mbuyi-Mayi, Kasai-Oriental, Democratic Republic of the Congo
9Clinical Biology, Kinshasa University Teaching Hospital, Democratic Republic of the Congo
*Address for Correspondence: Mbula Mambimbi Marcel, Infectious Diseases Service, Kinshasa University Teaching Hospital, Democratic Republic of the Congo, Tel: +243814526574; Email: [email protected]
Background and aim: Metabolic abnormalities are common in HIV/AIDS. Increasingly, lipid ratios are used as screening tools for dyslipidaemia in these medical conditions. The aim of this study was to assess the ability of 4 lipid ratios to predict cardiovascular risks.
Methods: This is a cross-sectional and analytical study included 105 HIV+ patients followed in Kinshasa University Teaching Hospital (KUTH). Four indices [Atherogenic Index of Plasma (AIP), Castelli Risk Index (CRI) I and II, Atherogenic coefficient (AC)] were compared. Statistical analyzis consisted of measuring frequencies and means, Student’s t-tests, ANOVA and Ficher’s exact test, and the calculation of the Kappa value.
Results: Lipid ratios predicted respectively the risk in 62% (AIP), 28.6% (CRI-I) and 23.8% (CRI-II). CRI-I and II were elevated, especially in women. The AIP appeared to be a better predictor than CRI-I and II to assess dyslipidaemia in general and the high-risk frequency. The cholesterol detected risk in 66.7% (Low HDL-C), 50% (High LDL-C), 38.9% (High TC and/or TG).
The atherogenic risk was higher with age, advanced WHO stage, HIV-TB, HBV-HCV co-infections, smoking and alcohol intake. Haemoglobin (Hb) and CD4 counts were low when the risk was high. Age ≥ 50 years, stage 4 (WHO), CD4s+ ≤ 200 cells/µL were independent factors associated with atherogenic risk.
Conclusion: Lipid ratios can be used as reliable tools for assessing cardiovascular risk of naïve HIV-infected patients who received HAART.
HIV infection is currently a chronic condition since the use of highly active antiretroviral therapy (HAART) [1, 2]. Infections in general [3] and HIV infection particularly, through immune activation and inflammation that characterize them, lead to dyslipidaemia, which is the cause of atherosclerosis [2,4]. HIV per se is responsible for the onset of this medical condition, but opportunistic infections (OIs), antiretroviral therapy, traditional atherogenic risk factors [smoking, hypertension, total cholesterol, decrease in HDL-C, increased TG, diabetes mellitus, family history of coronary artery disease (CAD)] and many other factors are implicated in the occurrence of atherosclerosis during HIV infection [4-6]. From the first descriptions of HIV/AIDS, heart attacks had been described: dilated cardiomyopathy, myocarditis, infectious endocarditis, pericarditis, pericardial effusion, tamponade, arterial hypertension [5]. Since the introduction of HAART in 1996, CAD including myocardial infarction has been observed frequently [7]. HAART, dyslipidaemia, and other traditional cardiovascular risk factors have been implicated in the development of cardiovascular complications [8,9]. In HIV infection, HDL hypocholesterolaemia, hypertriglyceridemia and significantly increased visceral fat were noted [10,11]. The decrease in HDL-C and the increase in TG are two factors which act synergistically and have been responsible for the occurrence of myocardial infarction [1]. HAART has reduced morbidity and mortality from HIV, thereby improving the patient quality of life. Nevertheless, the propensity of protease inhibitors (PIs), especially those of the first generation and other classes of ART, to induce dyslipidaemias and insulin resistance has been observed, although these results have not been observed and verified in all studies [12]. During HIV infection, the risk of CAD increases because the risk of atherosclerosis increases. New complications associated with HIV infection leading to chronic pathology causing cardiovascular disease and cancer are emerging [1]. The toxicity of the ARTs to which the patient is exposed generates metabolic disturbances and premature aging. These abnormalities related to HIV infection itself, ARTs (particularly PIS), restoration of health, body modifications and increased risk of cardiovascular disease, independent of traditional risk factors put strain on health systems especially in resource-limited areas where many patients are still reaching the stage of advanced HIV disease despite WHO recommendations that patients be treated immediately after testing (test and treat strategy). Providers in these regions, like those in regions with more resources, will increasingly have to deal with multimorbidity for several years. Health systems will need to organize the management and prevention of these medical conditions associated with HIV disease. It is therefore essential to assess the atherogenic risk. This evaluation can be carried out by various methods, in this case by scores such as the European Systematic Coronary Risk Evaluation (SCORE), the Framingham Risk Score (FRS), the Data on Adverse Events of Anti-HIV Drugs (DAD) Score to predict cardiovascular risks over 10 or 5 years [13-17]. Assessment can also be done by looking for subclinical atherosclerosis, obtained by measuring the thickness of the common carotid intima-media. This is done using Doppler ultrasound [17-19]. Currently, indices obtained from the lipid ratios are increasingly used. They predict cardiovascular risk for cardiovascular disease and can be applied effectively in PLWHIVs [20-23]. Very few African studies [20-24] have evaluated the cardiovascular risk during HIV infection. Africa has the highest number of PLHIVs and does not have the means to properly care for them given limited resources. As the life expectancy of PLWHIVs increases with HAART, providers should be aware of the cardiovascular complications of HIV and should include evaluation of risk factors and cardiovascular risk in clinical routine. The burden of non-communicable diseases associated with HIV is increasing, and there is a need to use non-biological factors in patient management and to predict cardiovascular risk in PLWHIVs. In addition to prevention, care, HAART, programs to fight HIV/AIDS in Africa must develop innovative approaches to deal with these new medical conditions. It is therefore of interest for us to take stock of these questions to which many providers are not prepared in the context of HIV and which have been little studied in Africa and DRC. It is in this context that this study was initiated. The aim of this study was to assess the cardiovascular risks of our patients by using indices derived from lipid ratios used as tools for screening and predicting cardiovascular risks.
This is a cross-sectional and analytical study that was carrying out in the Infectious Diseases Service, Department of Internal Medicine, KUTH, DRC. It covered a period of 6 years (from January 1, 2008 to December 31, 2014). It concerns a consecutive series of HIV positive patients followed in outpatient and hospitalization. The parameters of interest for this study are listed below.
The general characteristics of the study population [average age, sex, marital status (married, single, widower, divorced), profession, level of education, religion, socioeconomic level, ethnicity].
The lipid profile in general [normal and abnormal values of Total cholesterol (TC), Low Density Lipoprotein-Cholesterol (LDL-C), High Density Lipoprotein Cholesterol (HDL-C), Triglycerides (TG)].
The dyslipidaemia in general [Abnormalities of one of the lipid fractions (Hypo or HyperTC, hypo or hyperLDL-C, hypoHDL-C, hyperTG)].
The lipid ratios:
Atherogenic Index of Plasma (AIP) = log10 (TG/HDL-C).
Castelli Risk Index (CRI): CRI-I = TC/HDL-C; CRI-II = LDL-C/HDL-C.
Coefficient of Atherogenicity (CA) = TC-HDL-C/HDL-C).
The average values of the lipid balance and the atherogenic risk prediction indices in the groups were studied and the comparison of atherogenic risk according to the indices [AIP, CRI-I and II, AC].
The diagnosis concordance of high risk of atherogenicity between various indices.
Clinical characteristics according to atherogenic risk.
Biological characteristics as a function of atherogenic risk.
Factors associated with high atherogenic risk.
The abnormal values of the indices used in the study are listed in table 1.
| Table 1: Abnormal values of the Indices used in the calculation of cardiovascular risk. | ||
| N° | Indice | Abnormal Values of indices |
| 1 | CRI-I-Males | > 3.5 |
| 2 | CRI-I-Females | > 3.0 |
| 2 | CRI-II | > 3.3 |
| 4 | AC | > 3.0 |
| 5 | AIP | < 1 = low Risk; between 1 and 2.4 = Medium Risk; > 2.4 = High Risk |
| CRI-I: Castelli Risk Index I; CRI-II: Castelli Risk Index II; AC: Atherogenic Coefficient; AIP: Atherogenic Index of Plasma | ||
The reference values of biological parameters are listed in table 2.
| Table 2: Reference values for haematological and biochemical assessment. | ||
| N° | Variable | Reference Values |
| 1 | Hb | 12.5 to 15 g/dL for men and 10 to 15 g/dL for women |
| 2 | Ht | 38% to 52% for men and 32% to 45% for women |
| 3 | WBC | 4,000 to less than 10,000 cells/mm3 |
| 4 | LF: Neutrophils | 30% to 60% |
| Lymphocytes | 26% to 60% | |
| Eosinophils | 0% to 12% | |
| 5 | CD4s+ | From 410 to 1590 cells/mm3 |
| 6 | Urea | 10% to 42 mg% |
| 7 | Creatinine | 0.5% to 1.2 mg% |
| 8 | ASAT | 0 to 40 IU/L |
| 9 | ALAT | 0 to 45 IU/L |
| 10 | High TC | When value > 200 mg/dL |
| 11 | Hypertriglyceridaemia | When TG > 150 mg/dL |
| 12 | High LDL-C | When value > 130 mg/dL |
| 13 | Low HDL-C | if value < 40 mg/dL for men and < 50 mg/dL for women |
| Hb: Haemoglobin; Ht: Haematocrit; WBC: White blood Count; LF: Leucocyte Formula; CD4: Cluster of Differentiation four; ASAT: Aspartate AminoTranferase; ALAT: Alanine AminoTransferase; TC: Total Cholesterol; LDL-C: Low Density Lipoprotein Cholesterol; HDL-C: high Density Lipoprotein Cholesterol | ||
Statistical analysis
Data were analyzed using Excel 2010 and SPSS for windows software version 22.0. Descriptive statistics were used to calculate frequencies, means and standard deviations. The use of Student’s t tests and ANOVA made it possible to compare the means of 2 groups for the Student’s t test and of more than 2 groups for the ANOVA test. Fisher’s x2 and exact test compared the proportions. The diagnostic concordance of atherogenic risk prediction according to the indices was evaluated by calculating the kappa value. The factors associated with the elevated atherogenic risk were sought using logistic regression tests in univariate and multivariate analysis; calculations of OR and 95% confidence intervals were used to estimate the degree of association between high atherogenic risk and independent factors. p < 0.05 was the threshold of statistical significance for all the tests used.
Ethical considerations
As the data had been de-identified, the use of informed consent and an ethics committee was not required. The staff of the service enforced ethical rules when collecting and processing data. The study had respected the rules of confidentiality, justice, and charity of PLWHIVs during all the processes leading to the publication of this article.
The general characteristics of the study population are in table 3.
| Table 3: General characteristics of the study population. | ||
| Variables | Effective n = 105 | Percentage |
| Age (years) mean ± SD | 44.5 ± 11.5 | |
| Sex | ||
| Male | 40 | 38.1 |
| Female | 65 | 61.9 |
| Marital status | ||
| Maried | 45 | 47.4 |
| Divorced | 5 | 5.3 |
| Single | 30 | 31.6 |
| Widower | 15 | 15.8 |
| Profession | ||
| Unemployed | 40 | 47.1 |
| Civil servant | 15 | 17.6 |
| Junior company officer | 5 | 5.9 |
| Liberal profession | 25 | 29.4 |
| Level of study | ||
| Primary | 5 | 8.3 |
| Secondary | 35 | 58.3 |
| University level | 20 | 33.3 |
| Religion | ||
| Catholic | 30 | 42.9 |
| Revival churches | 35 | 50.0 |
| Other | 5 | 7.1 |
| Socioeconomic level | ||
| Medium | 35 | 87.5 |
| Low | 5 | 12.5 |
| Ethnicity | ||
| Kongo | 40 | 50.0 |
| Ngala | 15 | 18.8 |
| Luba | 15 | 18.8 |
| Swahili | 10 | 12.5 |
Only 38.8% of our patients had performed the lipid assessment (105 out of 270 patients).
Of the 105 patients in the study, 61.9% were female (Sex ratio: 2 F/1 M). The mean age was 44.5 ± 11.5 years. The majority were married (47.4%), without profession (47.1%), of secondary education (58.3%), attending revival churches (50%). The average socioeconomic level predominated (87.5%) for patients for which data was available and a high frequency of the Kongo ethnicity in half of the cases (Table 3).
Table 4 shows the lipid balance and the predictive indices of atherogenic risk in the groups studied. The mean total cholesterol ± SD is higher in women (138.8 ± 42.4 for men and 163.0 ± 59.6 for women; p = 0.027). More women than men experimented hypercholesterolemia (38.5% of women and 12.5% of men; p = 0.003). Low HDL-C levels are statistically high in women (53.8%) than in men (25.0 %; p = 0.003). In general, dyslipidaemia is more frequent in women (46.2%) than in men (25.0%), p = 0.024. Overall CRI-I and CRI-2 are high, and they are statistically higher in women than in men.
Dyslipidaemia in general had a frequency of 38.1% in the study group (Table 4).
| Table 4: Average values of lipid balance and predictive indices of atherogenic risk in the groups studied. | ||||
| Variables | All n = 105 | Male n = 40 | Female n = 65 | p |
| TC (mg/dl); | 153.8 ± 54.8 | 138.8 ± 42.4 | 163.0 ± 59.6 | 0.027 |
| LDL-C (mg/dl) | 79.5 ± 47.0 | 71.0 ± 38.9 | 84.8 ± 50.9 | 0.147 |
| HDL-C (mg/dl) | 60.2 ± 42.7 | 58.7 ± 31.3 | 61.1 ± 48.6 | 0.780 |
| TG (mg/dl) | 141.7 ± 61.0 | 135.1 ± 63.5 | 145.7 ± 59.6 | 0.386 |
| Hypercholesterolaemia | 30 (28.5) | 5 (12.5) | 25 (38.5) | 0.003 |
| Elevated LDL | 20 (19.0) | 5(12.5) | 15 (23.1) | 0.139 |
| Low HDL | 45 (42.9) | 10 (25.0) | 35 (53.8) | 0.003 |
| Hypertriglyceridaemia | 25(23.8) | 10 (25.0) | 15 (23.1) | 0.500 |
| Dyslipidaemia | 40 (38.1) | 10 (25.0) | 30 (46.2) | 0.024 |
| CRI-I | 3.8 ± 2.5 | 3.0 ± 1.7 | 4.3 ± 2.9 | 0.018 |
| CRI-II | 2.2 ± 1.7 | 1.6 ± 1.2 | 2.6 ± 1.9 | 0.008 |
| AC | 2.8 ± 2.6 | 2.0 ± 1.7 | 3.3 ± 2.9 | 0.180 |
| AIP | 0.44 ± 0.36 | 0.38 ± 0.29 | 0.48 ± 0.39 | 0.162 |
| Data are expressed as mean ± SD (standard deviation), absolute (n) and relative (in percent) frequency. Abbreviations: TC: Total Cholesterol; LDL-C: Low Density Lipoprotein Cholesterol; HDL-C: High Density Lipoprotein Cholesterol; TG: Triglycerides; CRI: Castelli Risk Index; AC: Atherogenic Coefficient; AIP: Atherogenic Index of Plasma |
||||
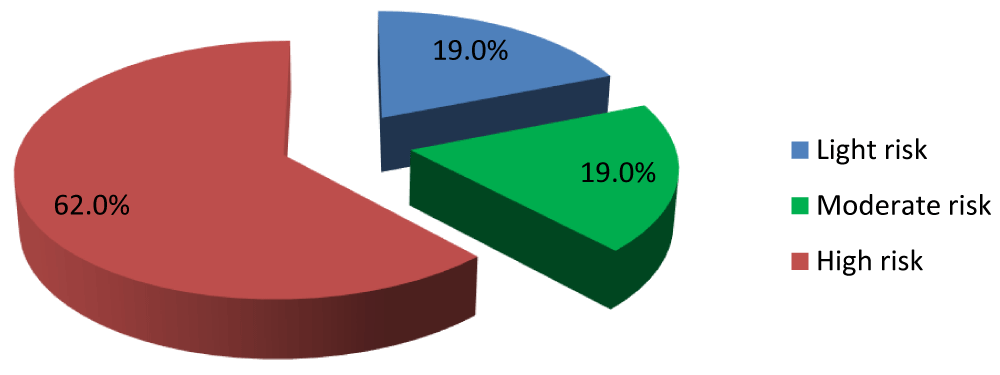
Risk of AIP atherogenicity in three categories
Figure 1 shows 62% of patients had a high atherogenic risk.
Figure 1: Frequency of atherogenic risk in the study population.
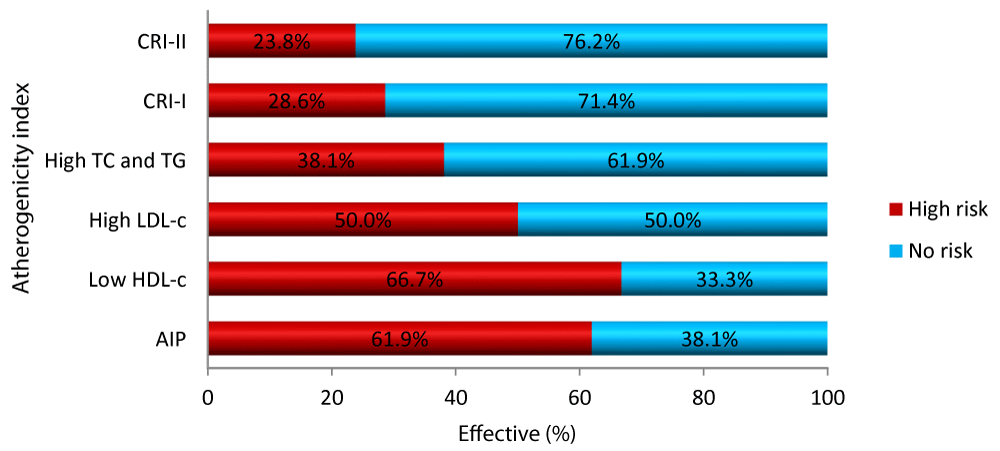
Comparison of atherogenic risk according to the lipid ratios and fractions
Figure 2 shows that the AIP and low HDL-C predicted a high frequency of risk compared to CRI-I, CRI-II and dyslipidaemia in general, but HDL-C scores better.
Figure 2: Atherogenic risk according to the lipid Ratios and fractions.
The high risk of atherogenicity according to the indices considered was completely consistent with the low HDL-C, CRI-I and CRI-II index (kappa = 1) on the one hand and fairly well concordant (kappa = 0.863) between TC and / or high TG and High LDL-C (kappa = 0.895) on the other hand (Table 5).
| Table 5: Diagnosis concordance of high atherogenicity risk between various indices. | |||
| AIP | |||
| Concordance | p - value | Kappa | |
| TC and/or Elevated TG | 75.0 | < 0.001 | 0.863 |
| CRI-I | 100.0 | < 0.001 | 1.00 |
| CRI-II | 100.0 | < 0.001 | 1.00 |
| AC | 100.0 | < 0.001 | 1.00 |
| Low HDL-C | 100.0 | < 0.001 | 1.00 |
| High LDL-C | 75.0 | < 0.001 | 0.895 |
Table 6 shows that the atherogenic risk significantly increased linearly with the age of the patients and is higher (p < 0.001) when patients are elder. The risk increased also with the WHO clinical stages of infection. In the other hand, the frequency of high risk was significantly increased in patients with a history of TB, with HBV-HCV coinfection, tobacco, and alcohol users (p < 0.05) and the frequency of fever was higher in patients with high atherogenic risk (p = 0.013). However, the frequency of weight loss decreased when the risk was high (p = 0.015). The mean of SBP and DBP was significantly higher in patients with high atherogenic risk. Patients with TB and anaemic PLWHIV had a significantly high frequency of elevated atherogenic risk (Table 6).
| Table 6: Clinical characteristics according to atherogenic risk. | |||||
| Variables | All n = 105 | Low Risk n = 20 | Medium Risk n = 20 | Elevated Risk n = 65 | p |
| Age (Years ± SD) | 44.5 ± 11.5 | 34.3 ± 9.8 | 43.0 ± 8.4 | 48.3 ± 10.8 | < 0.001 |
| < 50 | 70 (66.7) | 20 (100.0) | 15 (75.0) | 35 (53.8) | |
| ≥ 50 | 35 (33.3) | 0 (0.0) | 5 (25.0) | 30 (46.2) | |
| Sex | 0.155 | ||||
| Male | 40 (38,1) | 10 (50,0) | 10 (50,0) | 20(30,8) | |
| Female | 65 (61,9) | 10 (50,0) | 10 (50,0) | 35(69,2) | |
| Stage WHO | 0.030 | ||||
| 3 | 30 (28,6) | 15 (75,0) | 0 (0,0) | 15 (23,1) | |
| 4 | 55 (52,4) | 5 (25,0) | 20 (100,0) | 30 (46,2) | |
| Antecedents | |||||
| TB | 30 (28,6) | 0 (0,0) | 5 (25,0) | 25 (38,5) | 0.004 |
| Oral candidiasis | 15 (14,3) | 0 (0,0) | 5 (25,0) | 10 (15,4) | 0.084 |
| Co-infection HBV-HCV | 10 (11,1) | 0 (0,0) | 5 (33,3) | 5 (8,3) | 0,010 |
| HTA | 15 (14,3) | 0 (0,0) | 0 (0,0) | 15 (23,1) | 0.001 |
| Smoking | 10 (9,5) | 0 (0,0) | 0 (0,0) | 10 (15,4) | 0.010 |
| Alcohol | 20 (19,0) | 5 (25,0) | 0 (0,0) | 15 (23,1) | 0.023 |
| Clinical signs | |||||
| Fever | 80(76,2) | 10(50,0) | 15(75,0) | 55(84,6) | 0.013 |
| weight loss | 50(47,6) | 10(50,0) | 15(75,0) | 25(38,5) | 0.015 |
| Cough | 90(85,7) | 15(75,0) | 15(75,0) | 60(92,3) | 0.089 |
| Vomiting | 90(85,7) | 15(75,0) | 20(100,0) | 55(84,6) | 0.084 |
| Headache | 10(9,5) | 5(25,0) | 0(0,0) | 5(7,7) | 0.063 |
| Conjunctival pallor | 30(28,6) | 5(25,0) | 10(50,0) | 15(23,1) | 0.089 |
| SBP (mm Hg) | 124,2 ± 17.1 | 127,0 ± 15.5 | 108,8 ± 18.3 | 128,1 ± 14.6 | < 0.001 |
| DBP (mm Hg) | 79,3 ± 14.5 | 75,5 ± 17.6 | 72,2 ± 13.1 | 82,6 ± 12.9 | 0.008 |
| Respiratory rate (cpm) | 21,5 ± 4.9 | 26,0 ± 10.6 | 21,8 ± 1.3 | 20,3 ± 1.7 | < 0.001 |
| BMI (Kg/m2) | 24,8 ± 7.8 | 20,4 ± 4.1 | 24,2 ± 4.1 | 26,1 ± 8.7 | 0.126 |
| Comorbidities | |||||
| Tuberculosis | 30(28,6) | 0(0,0) | 5(25,0) | 25(38,5) | 0.001 |
| Oral candidiasis | 15(14,3) | 0(0,0) | 5(25,0) | 10(15,4) | 0.084 |
| Anaemia | 25 (23,8) | 0 (0,0) | 5 (25,0) | 20 (30,8) | 0.007 |
| Meningeal Cryptococcosis | 10 (9,5) | 5 (25,0) | 0 (0,0) | 5 (7,7) | 0.063 |
| Data are expressed as mean ± SD (standard deviation), absolute (n) and relative (in percent) frequency. Abbreviations: TB: Tuberculosis; HTA: Hypertension; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; BMI: Body Mass Index; cpm: cycle per minute; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus |
|||||
At high risk, Hb and CD4s+ levels decreased while the WBC count increased significantly when the risk was high (Table 7).
| Table 7: Biological characteristics and 3 lipid ratios as a function of atherogenic risk. | |||||
| Variables | All n = 105 | Low Risk n = 20 | Moderate Risk n = 20 | High Risk n = 65 | p |
| Hb (g/l) | 9.4 ± 2.9 | 10.3 ± 3.7 | 7.2 ± 2.5 | 9.6 ± 2.6 | 0.004 |
| Ht (%) | 30.3 ± 7.7 | 32.0 ± 5.3 | 29.0 ± 10.5 | 30.0 ± 7.7 | 0.596 |
| WBC (Cells/mm3) | 5847.6 ± 186.1 | 5200.0 ± 217.3 | 5133.3 ± 97.6 | 6278.2 ± 188.7 | 0.021 |
| Neutrophils (%) | 57.9 ± 12.9 | 65.8 ± 11.3 | 54.7 ± 13.2 | 56.1 ± 12.4 | 0.008 |
| Lymphocytes (%) | 38.1 ± 12.1 | 30.3 ± 8.3 | 40.0 ± 7.6 | 40.5 ± 13.1 | 0.003 |
| Glycaemia (mg/dl) | 96.8 ± 25.9 | 90.5 ± 24.5 | 88.0 ± 14.8 | 103.8 ± 28.3 | 0.102 |
| Urea (mg/dl) | 31.1 ± 7.9 | 26.6 ± 8.7 | 24.7 ± 3.9 | 34.2 ± 7.9 | 0.221 |
| Creatinine (mg/dl) | 1.37 ± 0.5 | 0.82 ± 0.08 | 0.99 ± 0.09 | 1.65 ± 0.8 | 0.053 |
| AST (UI/l) | 31.1 ± 21.1 | 29.2 ± 4.3 | 26.3 ± 17.4 | 32.9 ± 24.9 | 0.518 |
| ALT (UI/l) | 30.4 ± 18.3 | 35.4 ± 8.2 | 21.0 ± 15.3 | 31.1 ± 13.5 | 0.316 |
| CD4s+ (Cells/µL) | 229.5 ± 72.3 | 325.3 ± 68.8 | 209.5 ± 49.8 | 98.5 ± 48.7 | < 0.001 |
| TC (mg/dl) | 153.8 ± 54.8 | 157. 3± 48.4 | 160.8 ± 40.2 | 150.5 ± 60.6 | 0.733 |
| LDL-C (mg/dl) | 79.5 ± 47.0 | 26.7 ± 6.4 | 107.6 ± 47.8 | 87.2 ± 41.5 | < 0.001 |
| HDL-C (mg/dl) | 60.2 ± 42.7 | 128.9 ± 39.1 | 53.8 ± 9.2 | 41.0 ± 24.9 | < 0.001 |
| Triglycerides (mg/dl) | 141.7 ± 61.0 | 120.3 ± 21.4 | 83.9 ± 15.3 | 166.0 ± 63.7 | < 0.001 |
| CRI_I | 3.79 ± 2.58 | 1.23 ± 0.2 | 3.17 ± 1.2 | 4.7 6 ± 2.7 | < 0.001 |
| CRI_II | 2.21 ± 1.71 | 0.23 ± 0.07 | 2.09 ± 0.95 | 2.85 ± 1.69 | < 0.001 |
| AC | 2.79 ± 2.58 | 0.24 ± 0.2 | 2.17 ± 1.2 | 3.77 ± 2.69 | < 0.001 |
| Data are expressed as mean ± SD (Standard Deviation). | |||||
On univariate analysis, age ≥ 50 years, WHO stage, TB, anaemia, and CD4s+ < 200 cells/µL had emerged as factors associated with high atherogenic risk. After multivariate adjustment, age ≥ 50 years, WHO stage 4, TB, and CD4s+ count < 200 cells/µL persisted as an independent factor associated with high atherogenic risk. Age ≥ 50 years increased the risk of atherogenicity by 8 times, stage 4 by 3, TB by 5 and the CD4s+ count < 200 cells/µL by 10 (Table 8).
| Table 8: Factors associated with high atherogenic risk. | |||||
| Variables | Univariate Analysis | Multivariate Analysis | |||
| P | OR (IC 95%) | p | aOR (IC 95%) | ||
| Age (Years) | |||||
| < 50 | 1 | 1 | |||
| ≥ 50 | 0,001 | 6,00 (2.09-17.26) | 0,005 | 7,54 (1.85-10.77) | |
| Stage 4 WHO | |||||
| No | 1 | 1 | |||
| Yes | 0,002 | 2,27 (1.26-4.75) | 0,007 | 2.77 (1.17-4.76) | |
| TB | |||||
| No | 1 | 1 | |||
| yes | 0,006 | 4,38 (1.51-12.65) | 0,000 | 5,21(3.78-9.26) | |
| Anaemia | |||||
| No | 1 | 1 | |||
| Yes | 0,039 | 3,11(1.06-9.12) | 0,123 | 1,74 (0.69-3.74) | |
| CD4s+ (Cells/µL) | |||||
| ≥ 200 | 1 | 1 | |||
| < 200 | < 0,001 | 8,17 (2.84-23.49) | 0,001 | 9,93 (2.69-12.90) | |
This study evaluated the prediction of cardiovascular risk in HAART-naive HIV patients attending the KUTH using lipid ratios. The general characteristics and the lipid abnormalities observed in the study population have been described and discussed previously in another study. Dyslipidaemia was present even before the initiation of HAART [25]. In this study, lipid abnormalities were found more in women than in men (Statistically higher mean TC value, more frequent hypercholesterolemia, statistically significant low HDL-C, more frequent presence of dyslipidaemia). The reasons for this variation are not known. Lipid ratios (AIP, CRI-I, CRI-II and AC) have been studied as predictors of cardiovascular risk in this paper. Other authors have also used these tools to predict the atherogenic risk in HIV infection. [20-24,26] Apart from HIV infection, these indices have also been used to assess cardiovascular risk in pre-eclampsia, type 2 diabetes mellitus, in stroke, among university staff in Malaysia, in dwellers in Nigeria etc... [28-30,31]. In our study, comparisons between these indices have been made and have shown that they can predict this risk reliably. By comparing the 4 indices, AIP seems to be the best marker of atherogenic risk. AIP can be considered as an independent factor impacting on cardiovascular risk [21]. AIP has been found to be frequently abnormal in HAART-naïve PLWHIVs. It is recommended to make it an index to be calculated in a baseline assessment of PLWHIVs before starting HAART [22]. AIP, AC, CRI are predictors of emerging cardiovascular risk in treated PLWHIVs [23]. In this series, CRI-I and CRI-II were also statistically higher in females. These indices can therefore be calculated before the initiation of HAART and be used in the evaluation of cardiovascular risk apart from cholesterol and its fractions as well as AIP. Dyslipidaemia in general was only detected in 38.1% while with AIP the risk was predicted in 62% of cases. Nevertheless, CRI-I and CRI-II had a low predictive ability, even when compared to TC and/or high TG. In our series, the cardiovascular risk is high in the elderly, elderly women, people with advanced HIV disease. For various reasons, the diagnosis of HIV infection is made late in older adults. Hence the late initiation of HAART when their immune system deteriorates over time [32]. The reason why cardiovascular risk is higher in women with older age is not known. In advanced HIV disease, the risk is extremely high as immune activation and chronic inflammation are particularly important. PLWHIVs with TB and HBV-HCB co-infection, high blood pressure, smoking and alcohol use were also at high risk. These results support the recommendations of the National AIDS and STI Control Program (PNLS) which stipulate that tuberculosis must be actively sought out in PLHIVs in order to prevent and treat it. The health care providers should also check for HIV-hepatitis, HCV, and HBV co-infections. These comorbidities can have a negative impact on the health of PLWHIVs and eventually lead to serious complications. While collecting data on the history of the disease, providers should look for traditional cardiovascular risk factors that are otherwise frequently encountered in elderly PLWHIVs. A few clinical signs have played a predictive role in the onset of cardiovascular risk. These are fever, weight loss, elevated SBP and DBP, and respiratory rate. Fever in HIV infection is caused by different factors (HIV itself, infectious, cancerous, and other comorbidities). It therefore occurs in a context of immune activation and chronic inflammation and frequently at an advanced stage of the disease. Many PWLHIVs experience significant weight loss in the late stages of HIV infection. In a context where the number of elderly PLWHIVs is increasing, pathologies such as hypertension and diabetes will also increase. Hence the need to take vital signs at each consultation in order to monitor the appearance of hypertension if it is not already present and the weight, even if in the context of low resources, the equipment may be lacking in the care structures. In co-morbidities, TB and anaemia were medical conditions predicting high cardiovascular risk. In DRC, HIV infection occurs in a context of poverty, malnutrition, and low income for the population. It inevitably leads to anaemia. In the biological assessment, the blood count gave a statistically significant indication (Hb, WBC, neutrophils, lymphocytes). The blood count is among the basic examinations recommended by the PNLS. The CD4s+ count which gives us an idea of the immune status shows that the risk is high in advanced HIV disease. In univariate and multivariate analysis, this study shows that advanced age (from 50 years), advanced HIV disease (WHO Stage 4 and CD4s+ < 200 cells/µL) and TB were factors associated with high atherogenic risk. In univariate analysis alone, anaemia was also a factor associated with atherogenic risk. The results of this study indicate that health structures caring for PLWHIV must realize that they will increasingly have to manage medical conditions with patients with multimorbidities and polypharmacies. When collecting data on the history of affection, providers must increasingly consider personal and family cardiovascular risk factors (hypertension, smoking, alcohol intake, diabetes mellitus, history of CAD, obesity, hypercholesterolemia, etc). These data also give an indication to the PNLS on the importance of achieving the lipid profile. This means that providers need to be trained and their knowledge updated regularly. They should learn to estimate cardiovascular risk by lipid ratios. The lipid profile and lipid ratios must be part of the routine care of PLWHIVs. The PNLS must advocate to obtain funding to subsidize the biological examinations. CD4s+ counting remains relevant and funding must be obtained for this examination, because it allows to assess the immunological status at the initial treatment, but also to determine if the PLWHIV is already at an advanced stage of the disease. Although the number of patients was not large, this study gives an indication of the place of lipid ratios in the evaluation of cardiovascular risk in PLWHIVs in addition to the lipid profile.
Lipid ratios have been studied by other authors and we compare our results to theirs in table 9.
| Table 9: Comparison of some studies on lipid ratios. | ||||||||||||
| N° | Authors Year Reference [Ref] Location Nature of Study |
Sutdy Population | Age (years) (Mean ± SD) |
TC (mmol/L or mg/dL) |
LDL-C (mmol/L or mg/dL) |
HDL-C (mmol/L or mg/dL) |
TG mmol/L or mg/dL |
Glycaemia mmol/L or mg/dL |
AC TC-HDL-C/HDL-C |
CRI-I TC/HDL-C |
CRI-II LDL-C / HDL-C |
AIP Log10 (TC/HDL-C) |
| 1 | Oseghe, et al. 2016, [20] Lagos University Teaching Hospital Nigeria Cross-sectional study |
HIV-naïve patients =100 HIV-treated patients =100 HIV-negative controls = 83 |
HIV-naïve patients = 35.6 ± 8.1 HIV-treated patients = 35.5 ± 7.8 HIV-negative patients = 36.6 ± 7.2 |
HIV-naïve= 4.7 ± 1.4 HIV-treated = 5.5 ± 1.4 Controls = 5.1 ± 1.1 |
HIV-naïve 3.1 ± 1.2 HIV-treated = 3.3 ± 1.2 Controls = 3.3 ± 1.1 |
HIV naïve= 0.9 ± 0.4 HIV-treated = 1.5 ± 0.6 Controls = 1.4 ± 0.3 |
HIV-naïve= 1.4 ± 0.7 HIV-treated =2.0 ± 1.2 Controls = 1.5 ± 0.6 Controls = 1.4 ± 0.3 |
4.9 ± 1.6 | Not calcuted | HIV-naïve =5.9 ± 3.7 HIV-treated=4.8 ± 5.2 (CRI-I > 5.6 = High risk for CVD) |
HIV-naïve = 3;6 ± 2.5 HIV-treated = 2.5 ± 1.4 Range of 3.3 ± 3.7 (Increased risk of death for CVD) |
HIV-naïve = + 0.18 ± 0.3 HIV-treated=-0.03 ± 0.4 (More positive Ratio = more CVD risk) |
| 2 | Noumegni, et al. 2017. [21] HIV day-care unit of the Yaounde´ Central Hospital, University of Yaoundé, Cameroon. Cross-sectional study |
452 HIV patients. Aged 30 to 74 years. No history of CVD, not pregnant or breast-feeding women. Not on lipid-modifying therapy or hormone |
80% women. 44 ± 10 |
4.5 ± 1.0 | 23 ± 0.9 | 1.7 ± 0.6 | 1.0 ± 0.6 | 5.1_0.9 | Was not the subject of this study | Was not the subject of this study | Was not the subject of this study | AIP values Range: from - 0.63 to 1. (-0.08-0.31). High AIP: 32.5%. Median Risk of CVD: 2.11 (0.67-5.32) |
| 3 | Onyedum, et al. 2014. [22] University of Nigeria Teaching Hospital, Nigeria Cross-Sectional Study |
353 (70.8%) HAART-naïve patients |
Mean Age (Years) 37.3 ± 9.6 |
15.5% (Elevated TC) Mean ± SD 4.0(1.2) |
4.5 % (Elevated LDL-C) Mean ± SD 1.9(1.1) |
62.3 % (Low HDL-C): Most common abnormality) 0.8(0.7) |
35.4% (Elevated TG) Mean ± SD 1.5(0.8) |
Not dosed | Not calculated | CRI-I > 5 = 146 ± 41.4 33.7% with CRI-I >3 |
Not calculated | AIP range: from 1.0 to 1.9. Median (IQR): 0.3 (0.6) AIP > 0.24 = 52.1% |
| 4 | Noumegni, et al. 2017. [24] [Yaoundé Central Hospital (University Teaching Hospital, Cameroon)] Cross-Sectional Study |
452 HIV-Infected patients [361 women = (80%)]; 85%: on HAART |
44.4±10 (Ages: between 30 and 74 years) |
Mean ± SD mmol/L 4.5 ± 10 |
Mean ± SD mmol/L 2.3 ± 0.9 |
Mean ± SD mmol/L 1.7 ± 0.6 |
Mean ± SD mmol/L 1 ± 0.5 |
Mean ± SD mmol/L 5.1 ± 0.9 |
Not calculated | Not calculated | Not calculated | Median:0.11(-0.08-0.31); 32.5 % had a high AIP |
| 5 | Adedokun, et al. 2017. [23] Department of Biomedical Sciences, Lodoke. Akintolo University of Tchnology. Cross-sectional. |
80 patients. 40 HIV-treated (50% Male, 50% Female) 40 HIV Treatment-naïve (40 % male, 60 % female). Age and sex matched. |
Naïve: 37.22 ± 1.39 Treated: 33.35 ± 1.33 |
Naïve: 2.43 ± 0.04 Treated: 3.93 ± 0.07 |
Naïve: 0.60 ± 0.03 Treated 2.03 ± 0.08 |
Naïve: 0.85 ± 0.02 Treated: 1.06 ± 0.03 |
Naïve: 1.97 ± 0.05 Treated: 1.83 ± 0.03 |
Naïve: 2.06 ± 0.12 Treated: 2.78 ± 0.11 |
Naïve: 2.90 ± 0.09 Treated: 3.78 ± 0.11 |
Naïve: 0.73 ± 0.01 Treated: 1.98 ± 0.09 |
Naïve: 0.37 ± 0.01 Treated: 0.24 ± 0.01 |
|
| 6 | Bekolo, et al. 2014. [26] Nkonsamba Reginal hospital. Cameroon. Cross-sectional study |
114 participants. 72.8 females |
43 years (IQR: 36-51) |
Hyper TC: 29.8 % |
High LDL-C level (>130 mg/dl): 33.3% |
Low HDL-C (< 40 mg/dl): 18.4 % |
HyperTG (TG > 150 mg/dl: 51.8 % |
Not calculated | Not calculated | TC/HDL-C ≥ 5 : 16.7 % |
Not calculated | Not calculated |
| 7 | Mbula, et al. 2020. Kinshasa University Teaching Hospital Cross-sectional |
105 patients. Female: 61.9% |
44.5 ± 11.5 | 153.8 ± 54.8 Hyper TC: 28.5% |
79.5 ± 47.0 High LDL-C: 19.0 % |
60.2 ± 42.7 Low HDL-C: 42.9 % |
141.7 ± 61.0 Hyper TG: 23.8% |
88.9 ± 25.9 Reference: [25] |
2.8 ± 2.6 | 3.8 ± 2.5 | 2.2 ± 1.7 | 0.44 ± 0.36 High risk: 62 % |
Both Lipid ratios, CRI, CRII, and AC might be used as reliable and precise markers for diagnosis of high atherogenic risk among naive HIV patients from Kinshasa University Hospital.
Furthermore, age > = 50 years, Stage 4 WHO, tuberculosis, anaemia, and CD4s < 200 Cells/µL were identified as the most important significant and independent determinants of high atherogenic risk in those Congolese naive HIV patients.
- Deeks S, Lewin SR, Havlir DV. The end of AIDS infection as a chronic disease. The Lancet. 2013; 382: 1525-1533. PubMed: https://pubmed.ncbi.nlm.nih.gov/24152939/
- Serrano-Villar S, Gutiérrez F, Miralles C, Berenguer J, Rivero A, et al. Human Immunodeficiency Virus as a Chronic Disease: Evaluation and Management of Nonacquired Immune Deficiency Syndrome-Defining Conditions. Open Forum Infect Dis. 2016; 3: 097. PubMed: https://pubmed.ncbi.nlm.nih.gov/27419169/
- Pirillo A, Catapano AL, Norata GD. HDL in Infectious Diseases and Sepsis. In: von Eckardstein A., Kardassis D. (eds) High Density Lipoproteins. Handbook of Experimental Pharmacology. 2015; 224: 483-508. PubMed: https://pubmed.ncbi.nlm.nih.gov/25522999/
- Feingold KR, Grunfeld C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. [Updated 2019 Jan 8]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. PubMed: https://www.ncbi.nlm.nih.gov/books/NBK326741/
- Reis RP. Cardiovascular Risk in HIV-infected patient. Rev Port Cardiol. 2019; 38: 471- 472. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3964878/
- Currier JS, Hsue PY. The Role of Inflammation in HIV-Associated Atherosclerosis—One Size May Not Fit All. J Infect Dis. 2020; 221: 495-497. PubMed: https://pubmed.ncbi.nlm.nih.gov/31077267/
- Abutaleb A, Feinstein MJ. Coranary Artery Disease in HIV. 2020. https://www.acc.org/latest-in-cardiology/articles/2018/01/18/08/57/coronary-artery-disease-in-hiv
- Hsue PY, Waters DD. HIV infection and coronary heart disease: mechanisms and management. Nature Rev Cardiol. 2019; 16: 745–759. PubMed: https://pubmed.ncbi.nlm.nih.gov/31182833/
- Seecheran VK, Giddings SL, Seecheran NA. Acute coronary syndromes in patients with HIV. Coronary Artery Disease. 2017; 28: 166-172. PubMed: https://pubmed.ncbi.nlm.nih.gov/27845996/
- Raposo MA, Armiliato GN, Guimarães NS, Caram CA, Silveira RD, et al. Metabolic disorders and cardiovascular risk in people living with HIV/AIDS without the use of antiretroviral therapy. Rev. Soc. Bras. Med. Trop. 2017 50: 598-606. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0037-86822017000500598&lng=en
- Kumar S, Dhanawal. Central obesity & dyslipidemia in HIV patients on antiretroviral therapy. Indian J Med Res. 2018; 148: 366–368. PubMed: https://pubmed.ncbi.nlm.nih.gov/30665998/
- Cerrato E, Calcagno A, D'Ascenzo F, et al. Cardiovascular disease in HIV patients: from bench to bedside and backwards. Open Heart. 2015; 2: e000174. PubMed: https://pubmed.ncbi.nlm.nih.gov/25815207/
- Policarpo S, Rodrigues T, Moreira AC, Valadas E. Cardiovascular risk in HIV-infected individuals: A comparison of three risk prediction algorithms. Rev Port Cardiol. 2019; 38: 463-470. PubMed: https://pubmed.ncbi.nlm.nih.gov/31522936/
- Touloumi G, Kalpourtzi N, Papastamopoulos V, et al. Cardiovascular risk factors in HIV infected individuals: Comparison with general adult control population in Greece. PLoS One. 2020; 15: e0230730. PubMed: https://pubmed.ncbi.nlm.nih.gov/32226048/
- Krikke M, Hoogeveen RC, Hoepelman AI, Visseren FL, Arends JE. Cardiovascular risk prediction in HIV-infected patients: comparing the Framingham, atherosclerotic cardiovascular disease risk score (ASCVD), Systematic Coronary Risk Evaluation for the Netherlands (SCORE-NL) and Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) risk prediction models. HIV Medicine. 2016; 17: 289-297. PubMed: https://pubmed.ncbi.nlm.nih.gov/26268806/
- Bittencourt MS. Estimating Cardiovascular risk in HIV-Infected Patients. Arq Bras Cardiol. 2020; 114: 76–77. PubMed: https://pubmed.ncbi.nlm.nih.gov/32049173/
- Silva AG, Paulo RV, Silva-Vergara ML. Subclinical Carotid Atherosclerosis and Reduced DAD Score for Cardiovascular Risk Stratification in HIV-Positive Patients. Arq Bras Cardiol. 2020; 114: 68-75. PubMed: https://pubmed.ncbi.nlm.nih.gov/31664317
- Schoepf IC, Buechel RR, Kovari H, Hammoud DA, Tarr PE. Subclinical Atherosclerosis Imaging in People Living with HIV. J Clin Med. 2019; 8: 1125. PubMed: https://pubmed.ncbi.nlm.nih.gov/31362391/
- Hanna DB, Guo M, žková PB, et al. HIV Infection and Carotid Artery Intima-media Thickness: Pooled Analyses Across 5 Cohorts of the NHLBI HIV-CVD Collaborative. Clin Infect Dis. 2016; 63: 249–256. PubMed: https://pubmed.ncbi.nlm.nih.gov/27118787/
- Osegbe D, Soriyan OO, Ogbenna AA, Okpara HC, Azinge EC. Risk factors and assessment for cardiovascular disease among HIV-positive patients attending a Nigerian tertiary hospital Ifeyinwa. Pan African Med J. 2016; 23: 206. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4907765/
- Noumegni SR, Nansseu JR, Bigna JJ, et al. Atherogenic index of plasma and 10-year risk of cardiovascular disease in adult Africans living with HIV infection: A cross-sectional study from Yaoundé, Cameroon. JRSM Cardiovasc Dis. 2017; 6: 2048004017740478. PubMed: https://pubmed.ncbi.nlm.nih.gov/29435266/
- Onyedum CC, Young EE, Iroezindu MO, Chukwuka CJ, Nwagha UI. Atherogenic index of plasma in highly active antiretroviral therapy-naïve patients with human immunodeficiency virus infection in Southeast Nigeria. Indian J Endocrinol Metab. 2014; 18: 631-636. PubMed: https://pubmed.ncbi.nlm.nih.gov/25285278/
- Adedokun AK, Olisekodiaka MJ, Adeyeye DA, et al. Castelli Risk Index, Atherogenic Index of Plasma, and Atherogenic Coefficient: Emerging Risk Predictors of Cardiovascular Disease in HIV-Treated Patients. Int J Clin Trials Case Stud. 2017; 2: 8-15.
- Noumegni, SR, Ama VJM, Assah, FK. et al. Assessment of the agreement between the Framingham and DAD risk equations for estimating cardiovascular risk in adult Africans living with HIV infection: a cross-sectional study. Trop Dis Travel Med Vaccines. 2017; 3: 12. PubMed: https://pubmed.ncbi.nlm.nih.gov/28883982/
- Mbula MMK, Situakibanza HNT, Lelo GM, Mbenza bl, Makulo JRR, et al. Lipid profile of antiretroviral therapy-naive HIV-infected patients attending infectious diseases service of University Teaching Hospital of Kinshasa, Democratic Republic of the Congo (DRC). Int J Clin Virol. 2020; 4: 102-108. https://www.microbiochemjournal.com/fulltext/hjcv/ijcv-aid1023.php
- Bekolo CE, Nguena MB, Ewane L, Bekoule PS, Kollo B. The lipid profile of HIV-infected patients receiving antiretroviral therapy in a rural Cameroonian population. BMC Public Health. 2014; 14: 236. PubMed: https://pubmed.ncbi.nlm.nih.gov/24606888/
- Singh M, Pathak MS, Paul A. A Study on Atherogenic Indices of Pregnancy Induced Hypertension Patients as Compared to Normal Pregnant Women. J Clin Diagn Res. 2015; 9: BC05-BC8. PubMed: https://pubmed.ncbi.nlm.nih.gov/26393117/
- Nimmanapalli HD, Kasi AD, Devapatla PK, Nuttakki V. Lipid ratios, atherogenic coefficient and atherogenic index of plasma as parameters in assessing cardiovascular risk in type 2 diabetes mellitus. Int J Res Med Sci. 2016; 4: 2863-2869.
- Bo MS, Cheah WL, Lwin S, New TM, Win TT, Aung M. Understanding the Relationship between Atherogenic Index of Plasma and Cardiovascular Disease Risk Factors among Staff of an University in Malaysia. J Nutrit Metabol. 2018; Article ID 7027624; 6.
- Sujatha R, Kavitha S. Atherogenic indices in stroke patients: A retrospective study. Iran J Neurol. 2017; 16: 78-82. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5526781/
- Olamoyegun MA, Oluyombo R, Asaolu SO. Evaluation of dyslipidemia, lipid ratios, and atherogenic index as cardiovascular risk factors among semi-urban dwellers in Nigeria. Ann Afr Med. 2016; 15: 194–199. PubMed: https://pubmed.ncbi.nlm.nih.gov/27853034/
- HIV info.NIH.gov. HIV and older people. 2020. https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-and-older-people